
Discover more from Sense of Awareness
NIACIN AND THE HEART
Quick overview why you want to take niacin (nicotinic acid) for a healthy heart, especially if you got the COVID19 shot or been exposed to its harm.
Niacin and GPR109A
Niacin and GPR109A have an interesting and complex interaction, playing a key role in various physiological processes, particularly in lipid metabolism and cardiovascular health. Here's a deeper dive into their interaction:
Binding Mechanism:
Niacin directly binds to the GPR109A receptor, specifically targeting residues like R111 on transmembrane helix 3 (TMH3).
This binding triggers a cascade of cellular responses, depending on the cell type and context.
Effects of Niacin-GPR109A Interaction:
Lipid Metabolism: Niacin activation of GPR109A inhibits lipolysis (fat breakdown) in adipose tissue, leading to decreased blood triglyceride and LDL cholesterol levels while increasing HDL cholesterol (good cholesterol). This contributes to its role in managing dyslipidemia and reducing cardiovascular risk.
Anti-inflammatory: GPR109A activation by niacin has anti-inflammatory effects, potentially reducing inflammation in various tissues, including the heart. This could be beneficial in mitigating chronic inflammatory conditions like atherosclerosis.
Other Effects: Depending on the context, niacin-GPR109A interaction may also influence energy metabolism, cell survival, and other processes, but the exact mechanisms are still being explored.
GPR109A and the heart
GPR109A, also known as hydroxycarboxylic acid receptor 1 (HCAR1), plays a multifaceted role in the heart, influencing various processes with potential implications for cardiovascular health. Here's a breakdown:
Main Functions:
Inflammation: GPR109A acts as an anti-inflammatory receptor, reducing inflammatory responses in the heart. This is particularly relevant in conditions like atherosclerosis, where chronic inflammation contributes to plaque buildup and heart disease.
Metabolic Regulation: The receptor interacts with various metabolites like niacin (vitamin B3) and 3-hydroxybutyrate (a ketone body), influencing energy metabolism and fatty acid utilization in the heart. This can offer potential benefits for improving energy efficiency and reducing heart strain.
Cardioprotection: Studies suggest GPR109A activation may offer cardioprotective effects, potentially reducing heart cell death and damage from ischemia (reduced blood flow) and other stressors.
Vascular Function: The receptor is expressed in blood vessel cells and might play a role in regulating blood vessel relaxation and contraction, impacting overall blood pressure and circulation.
Potential Implications:
Treating Cardiovascular Disease: The anti-inflammatory, metabolic, and cardioprotective properties of GPR109A make it a promising target for developing new therapies for various cardiovascular diseases like atherosclerosis, heart failure, and ischemia.
Modulating Metabolism: Understanding how GPR109A affects metabolism in the heart could open avenues for designing interventions to improve heart health by regulating energy usage and fatty acid metabolism.
Personalized Medicine: Studying individual variations in GPR109A activity or response to its ligands could help tailor treatments for cardiovascular diseases based on a patient's specific genetic makeup and response mechanisms.
GPR109A and the mitochondria
GPR109A and mitochondria have a fascinating and multifaceted relationship, with potential implications for understanding and treating various health conditions. Here's a breakdown of their connection:
Indirect Interactions:
Metabolic Regulation: GPR109A interacts with several metabolites that fuel the mitochondria, particularly 3-hydroxybutyrate (a ketone body) and niacin. By influencing these metabolites, GPR109A indirectly affects mitochondrial function. For example, activating GPR109A with niacin can increase ketone body production and decrease fat breakdown, ultimately influencing how mitochondria utilize these fuel sources.
Mitochondrial Stress: Studies suggest GPR109A activation might offer protection against mitochondrial stress and damage. This could be linked to its anti-inflammatory effects, preventing inflammation-induced mitochondrial dysfunction. However, the exact mechanisms are still being explored.
Potential Direct Link:
New Research: Emerging evidence suggests a possible direct link between GPR109A and mitochondria. Some studies indicate the receptor might be physically located on the mitochondrial outer membrane in certain cell types. This raises exciting possibilities for its role in directly impacting mitochondrial function and signaling.
Unanswered Questions: Despite these hints, the potential direct link between GPR109A and mitochondria remains largely unexplored. More research is needed to understand if and how the receptor directly interacts with mitochondria, what specific pathways are involved, and the implications for various physiological processes.
Nicotinic acid and the mitochondria
While nicotinic acid (niacin) has numerous effects on the body, its relationship with mitochondria is complex and multi-faceted. Here's a breakdown of what we know and what's still being explored:
Direct vs. Indirect Interactions:
Indirect: As a precursor to the essential molecule NAD+, nicotinic acid indirectly impacts mitochondrial function by influencing this key player in energy metabolism. NAD+ serves as a coenzyme in various mitochondrial processes, including the electron transport chain (ETC) responsible for ATP production. Increasing NAD+ levels through nicotinic acid can potentially enhance mitochondrial function and ATP production.
Possible Direct Effects: Some suggest a direct interaction between nicotinic acid and mitochondria due to:
GPR109A receptor: Nicotinic acid activates the GPR109A receptor, which might be located on the mitochondrial outer membrane in certain cell types. This potential direct link, however, still requires further investigation.
Antioxidant properties: Nicotinic acid exhibits antioxidant activity, potentially protecting mitochondria from free radical damage, thereby improving their function.
Potential Benefits:
Improved Energy Production: Enhanced mitochondrial function and ATP production due to increased NAD+ could benefit tissues with high energy demands, such as the heart, muscles, and brain.
Reduced Oxidative Stress: Nicotinic acid's antioxidant properties might protect mitochondria from free radical damage, potentially mitigating age-related decline and improving mitochondrial health.
Cardioprotection: Studies suggest nicotinic acid's impact on NAD+ and GPR109A activation might offer cardioprotective effects, but the specifics require further research.
COVID19 causes DNA damage:
There is growing evidence that COVID-19 infection can cause DNA damage in human cells. While the full extent and implications are still being studied, here's what we know so far:
Mechanisms of DNA damage:
Direct Viral Effects: The SARS-CoV-2 virus may directly damage DNA through mechanisms like:
Viral proteins: Some viral proteins can directly cause DNA breaks or interfere with DNA repair processes.
Oxidative stress: The virus can trigger excessive reactive oxygen species (ROS) production, leading to DNA damage.
Indirect Effects: The body's inflammatory response to the virus can also indirectly damage DNA through:
Chronic inflammation: Persistent inflammation can create an environment conducive to DNA damage.
Immune cell activity: Activated immune cells can generate ROS and other molecules that can damage DNA.
Evidence of DNA damage:
Studies: Research has shown increased levels of DNA damage markers in cells from COVID-19 patients compared to healthy individuals. These markers include DNA breaks, micronuclei, and DNA repair foci.
Potential consequences: DNA damage can have various consequences, including:
Increased risk of mutations: Mutations can lead to cancer and other diseases.
Cellular senescence: Damaged cells may stop dividing and contribute to tissue aging.
Impaired immune function: DNA damage can weaken the immune system's ability to fight off infections.
Nicotinic Acid and DNA repair
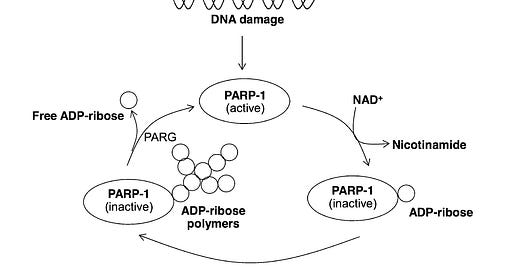
According to the article “Nicotinic acid and derivatives as multifunctional pharmacophores for medical applications” (Sinthupoom et al, 2015), Nicotinic acid, through its metabolite NAD+, plays a crucial role in both cellular energy production (ATP) and DNA repair via the activation of poly ADP-ribose polymerase (PARP-1). This enzyme, specifically PARP-1, serves as a key sensor and responder to DNA damage, recognizing and binding to single or double-strand breaks.
One crucial member of this crew is NAD+, a molecule powered by vitamin B3 (nicotinic acid). Think of NAD+ as fuel for the cell and an activator for a repair enzyme called PARP-1.
When the DNA blueprint gets a tear:
PARP-1 jumps in, grabbing molecules and attaching them to the damage like tags.
These tags act as signals, attracting other repair tools to mend the broken DNA.
Usually, the repair is successful, and the cell lives on.
But sometimes the tear is too big:
Fixing it can drain the cell's energy (like fuel for the repair tools).
This can lead to a different kind of cell death (Necrosis rather than apoptosis).
Here is a table that describes the differences:
In short, vitamin B3 might be a helping hand:
It helps make more NAD+, potentially boosting repair even in tough situations.
This could change how badly damaged cells die, making it less harmful.
Studies suggest that vitamin B3 (nicotinic acid) does more than just fuel our cells. Its metabolite, NAD+, might play a key role in fixing damaged DNA and keeping our genetic material stable.
Here's how:
Boosting repair power: Research shows that giving cells extra NAD+ before damaging their DNA (think of it like a pre-emptive strike) helped them repair the damage more effectively.
Redox reactions: Inside cells, NAD+ acts like a tiny electron carrier, involved in essential reactions that keep things running smoothly.
More than just energy: While NAD+ provides energy, it can also directly influence DNA repair by breaking apart and releasing specific molecules needed for the process.
Beyond DNA: Interestingly, vitamin B3 also influences fat metabolism and may even form beneficial compounds with metals in our bodies.
These are FEW of the reasons why everyone who got the shot (or not) should seriously consider taking niacin (nicotinic acid).
DISCLAIMER: I’ve used Google’s Gemini to generate the content above, including the summary of the academic paper.
Until the next now,
Ehden











So if I didn’t get The Shot, do I still need it? I should be taking a multivitamin. I do take a D every night before bed. Heart and liver issues.
I wanted to find out the difference between niacin-aka-nicotinic acid and niacinamide-aka-nicotinamide. This quote explained at least one difference: Niacin is used for support for healthy cholesterol levels already within the normal range. Niacinamide is used for support for joint comfort, support for sugar balance and support for brain function, but does not support cholesterol balance. There may be more differences especially since the niacin flush that only comes from real niacin shows up in the exact areas not covered by bathing suits.