#COptiGate - Commenting the FDA
My submission of the comment to the FDA, regarding the planned approval of the Covid-19 vaccines for children 6 months to 4 years old.
AS THE REGULATOR DID NOT PERFORM RISK ASSESSMENT OF THE CODON OPTIMIZATION TECHNOLOGY USED IN COVID 19 VACCINES, I object to the approval of the COVID19 vaccines for children between the ages of 6 months to 4 years old.
Inconsequential (“silent”) mutations are known as synonymous mutation.
Synonymous mutations contribute to cellular processes which are determining protein structure and function.
Synonymous mutations influence protein folding.
Protein misfolding has been linked with neurodegeneration in Alzheimer and Parkinson disease, and many other pathologies.
Protein misfolding resulting in intracellular PAO accumulation is sufficient to cause cardiomyocyte death and heart failure.
These misfolded proteins (PRIONS), cause a number of rare brain disorders, such as kuru, bovine spongiform encephalopathy (“mad cow” disease), and Creutzfeldt-Jakob disease.
The last decade has seen an exponential increase in published research on the topic of synonymous mutations.
Most studies that linked synonymous mutations to diseases focused on a single (or very few) nucleotide* substitutions.
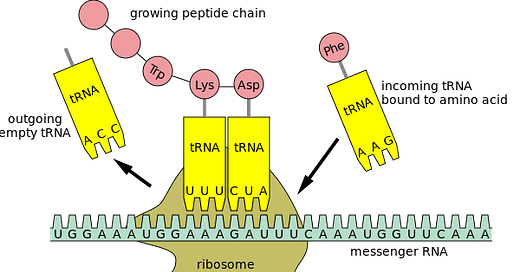
Codon optimization creates a large-scale alteration of the gene sequence.
Up until recently, codon optimization technology was only used for research and in the manufacturing of therapeutic proteins.
Pfizer, Moderna, AstraZeneca, Janssen etc. all use codon optimization, on top of other elements; their products do not generate WT SARS-CoV-2.
There is a lack of understanding on the difference between proteins synthesized using codon optimization compared to those which did not pass a codon optimization process.
Codon optimized proteins carry potential risks as their modified nucleotide sequences may have unpredictable effects on the structure and function of proteins.
Codon optimization can achieve efficiency in creating large amounts of the required protein (eg spike), but doing so at the expense of the accuracy of the protein being manufactured, resulting in generating unknown proteins due to errors/misreading.
Codon optimization might change the shape of the protein, which in result will lead the immune system to be trained to detect a wrong pathogen thus greatly reducing its ability to stop the pathogen, in our case SARS_CoV_2 and its mutants.
S-2P is an unstable protein.
S-2P's receptor binding domains (RDBs) are in an up (open) state, which doesn't train the body correctly as the original spike RDBs are closed.
Not only the S-2P design of the spike protein generated by Moderna, Pfizer and Janssen is faulty, introducing codon optimization on top of that might lead to errors in the RBD domain on the generate spike protein can further increase the risk of ADE.
The reason for the S-2P prefusion design was "to provoke stronger immune response...but it can also means the spike protein lingers in the plasma membrane bound to ACE2 receptors because of impaired fusion capabilities"
Antibody production against S-2P spike bind with soluble /plasma ACE-2 (shedding) can cause: coryzal symptoms; shortness of breath; abnormal liver function; LOST OF TASTE; Lymphopenia; Renal failure; ARDS; Kawasaki; myocarditis; and stoke.
It is therefore necessary to have well qualified methods that are fit-for-purpose and can be used to evaluate the risk of codon optimization during drug development and manufacture. The regulators did not have such a framework in place.
Prior to COVID19, the use of gene therapy was extremely limited, so even though regulatory agencies were well aware of the need for methods and processes, the limited use and the long review process deemed sufficient to identify codon optimization side effects.
Because in 2020 the WHO has declared COVID19 as a pandemic, the medical regulators have been instructed to get vaccines approved as fast as possible.
All the gene therapies which were introduced as vaccines to the SARS_CoV_2 virus and it's variants went via a fast-track process such as the FDA's Emergency Use Authorization (EUA)
The use of the fast-track approval process created a gap where the assumption that codon optimization side effects will be able to be identified as part of the review process, as there was no regulatory process to review risks related to codon optimization.
HOWEVER, since the fast-track process has been chosen, the risks which were usually being measured as part of the normal process were not measured.
The codon optimization related risks are not even required to be measured in the FDA's BLA for Pfizer vaccine.
The fact manufacturers use a technology which they are fully aware of is problematic, and the fact regulators are fully aware of it but do not even have a process to review it, puts in question the validation process and it's value.
NOT MEASURING A RISK DOES NOT MAKE IT GO AWAY, JUST MAKES IT INVISIBLE.
YOU MUST NOT EXPOSE KIDS TO RISKS BY PRETENDING THEY DO NOT EXIST.
Ehden Biber
(I enclosed PDF versions of the 4 #COptiGate threads)
Please go to the FDA website to submit your comment.
Subscribe to Sense of Awareness
The truth shall set you free...









and (sadly) nobody will care.
Excellent! Thank you.