The Rats
THE ULTIMATE PROOF THAT Pfizer/BioNTech NEVER DEMONSTRATED THEIR COVID19 "VACCINES" ARE SAFE OR EFFECTIVE, AND HOW HEALTH REGULATORS AROUND THE WORLD COLLUDED WITH THEM.
By Ehden Biber
This image holds the clue to what you are about to discover:
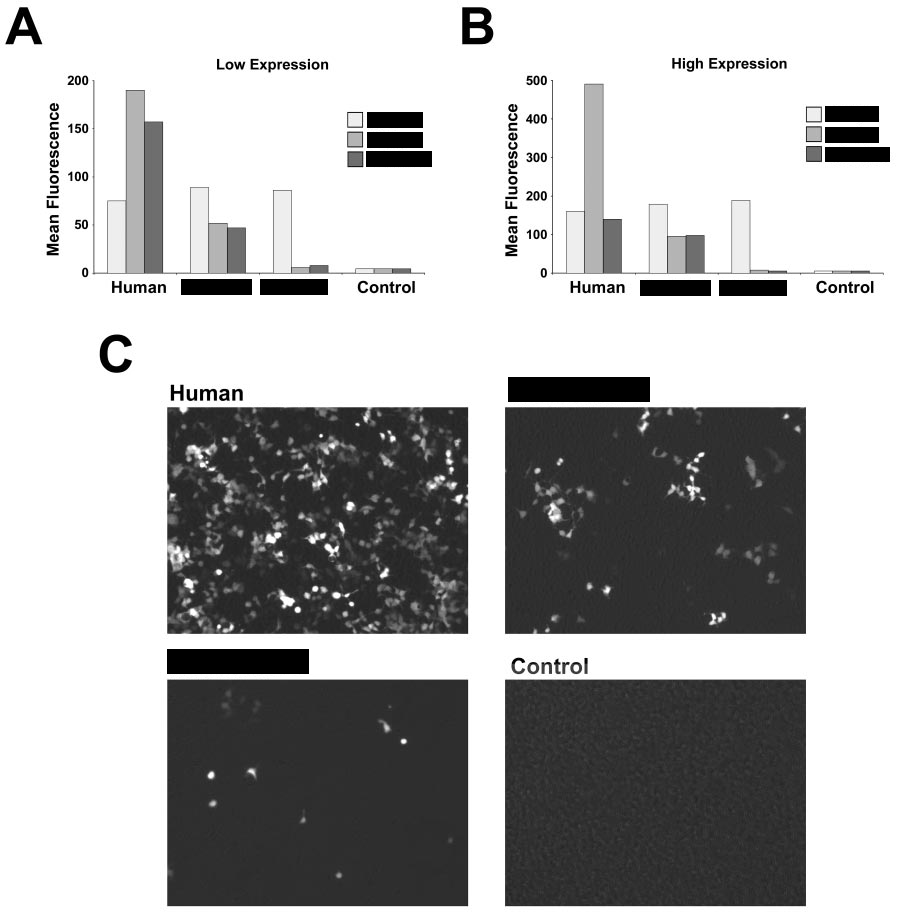
As you saw in the (sanitised) image above, humans seem to have a different expression of something. But of what? and what does it had to do with Pfizer/BioNTech experimental gene therapy, known as BNT162b2/Comirnaty/tozinameran?
Let's find out.
The Missing Studies
Pfizer/BioNTech didn't conduct many safety studies on animals prior to them receiving their Emergency Usage Authorization.
Genotoxicity & carcinogenicity studies were not performed, because "The…vaccine formulation…(was) not expected to have genotoxic potential". 🤨
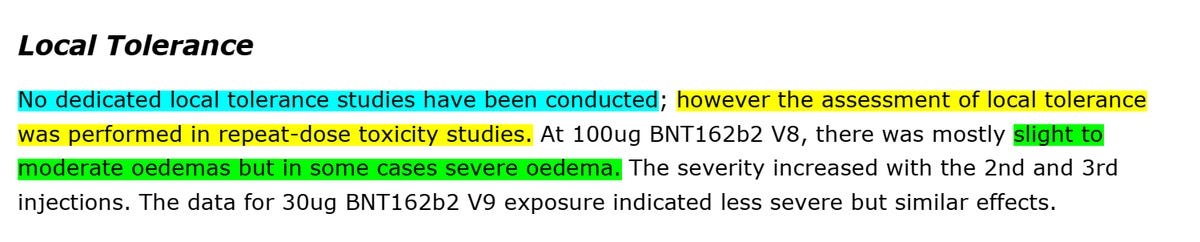
However, Pfizer/BioNTech stated that "the assessment of local tolerance was performed in repeat-dose toxicity studies" and that the only thing they saw was oedema. We will be back to this statement soon.
Pfizer/BioNTech didn't perform ecotoxicity / environmental risk assessment. The 2019 version of the NIH Guidelines on Research Involving (m)RNA Molecules lacked an appendix w/specific guidance on it, and it was a guidance, NOT a requirement. As the FDA stated, “The 2019 version of the National Institutes of Health (NIH) Guidelines on Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines) lacks an appendix providing specific guidance for institutional biosafety committee (IBC) review of clinical trials.”
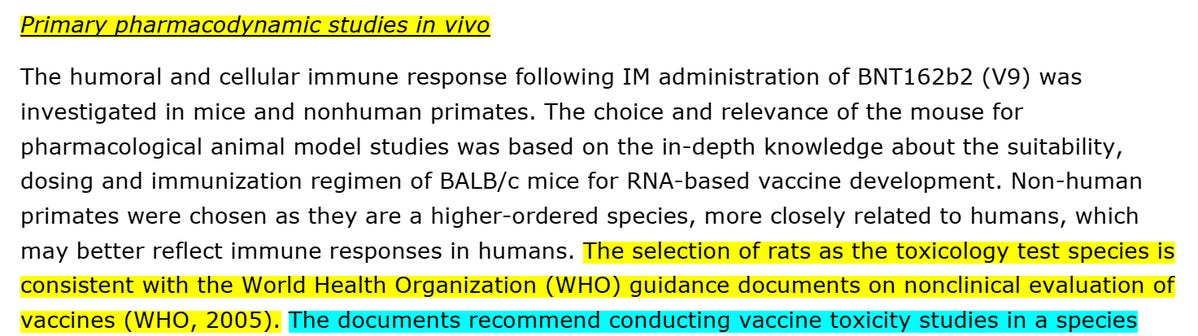
Pfizer/BioNTech didn't perform safety pharmacology studies. They claimed it is "not considered necessary according to the WHO guideline (WHO, 2005)", which refers to a WHO “guideline on nonclinical evaluation of vaccines, WHO Technical Report Series, No. 927, 2005”. According to this guidance, the testing of Pfizer/BioNTech "vaccine", a product that had no prior nonclinical and clinical experience, was "expected to be more extensive than for those vaccines previously licensed and used in humans".
Pfizer/BioNTech got exemption using the pink highlighted text from page 55 in the WHO 2005 guide, according to "The Vaccine: Inside the Race to Conquer the COVID-19 Pandemic" by Joe Miller, Özlem Türeci & Ugur Sahin. But…compare page 55 and what BioNTech claimed!
The Studies that were performed
So … that was the list of the studies that Pfizer/BioNTech DID NOT perform. Now, let's look at the tests they did perform, which they submitted to the regulators as a proof of the efficiency and safety of their product. Here it is where it gets REALLY interesting.
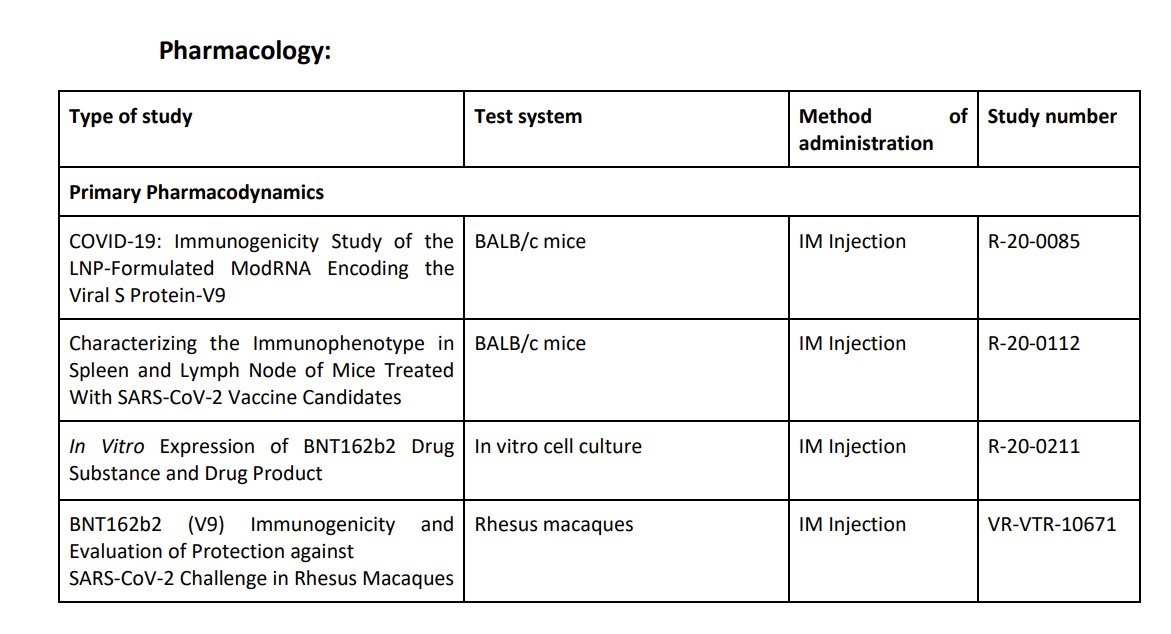
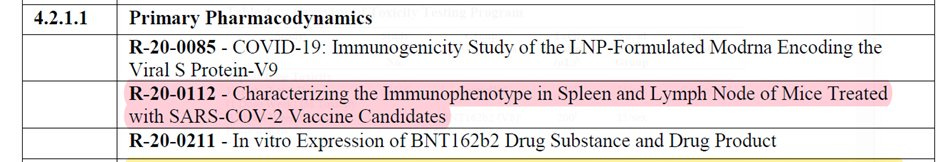
In total, Pfizer/BioNTech submitted four pharmacology studies in the pre-clinical phase: two studies (R-20-0085 and R-20-0112) involved mice, one (VR-VTR-10671) used rhesus macaques, and the fourth study (20-0211) was an in vitro study.
Pfizer/BioNTech performed immune and cellular immune response tests on 2 types of lab animals:
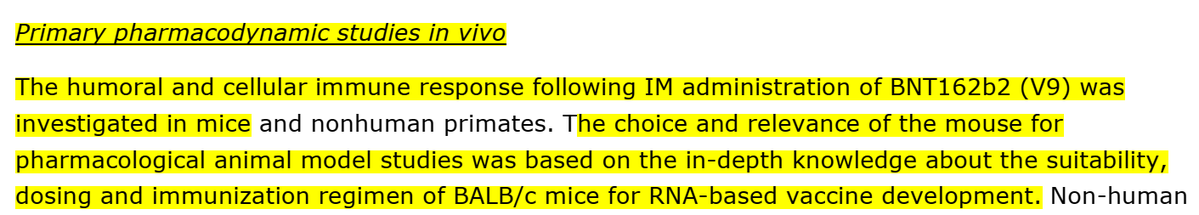
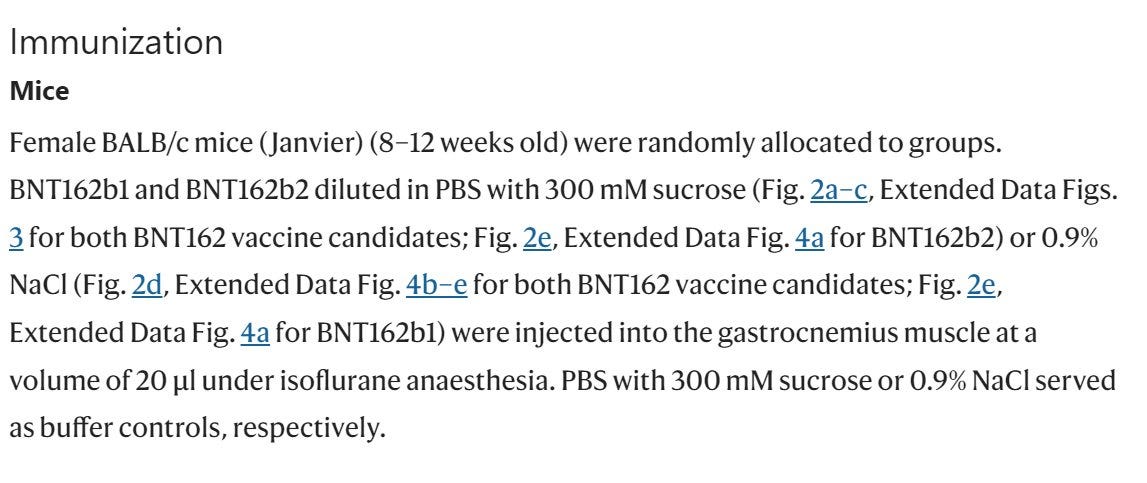
Mice: BALB/c mice (type of a lab mice) were used for "pharmacological animal model studies" and "for RNA-based vaccine development"
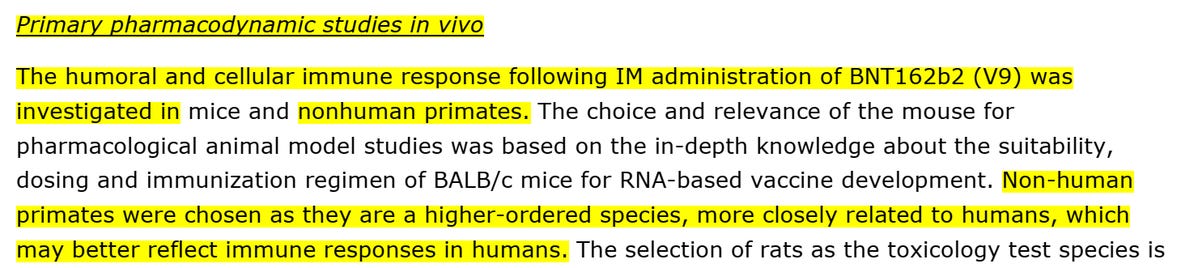
Rhesus macaques monkeys - the "non-human primates were chosen as they are a higher-ordered species, more closely related to humans, which may better reflect immune responses in humans". They used these monkeys for the immune and cellular immune response tests.
Rats: "in accordance with the…WHO…guidance documents on nonclinical evaluation of vaccines (WHO, 2005)…(used) The Wistar Han (WH) rat (who) developed an antigenspecific immune response following BNT162b2 vaccination".
According to their EMA submission "The choice and relevance of the mouse for pharmacological animal model studies was based on the in-depth knowledge about the suitability, dosing and immunization regimen of BALB/c mice for RNA-based vaccine development".
The Mice Studies
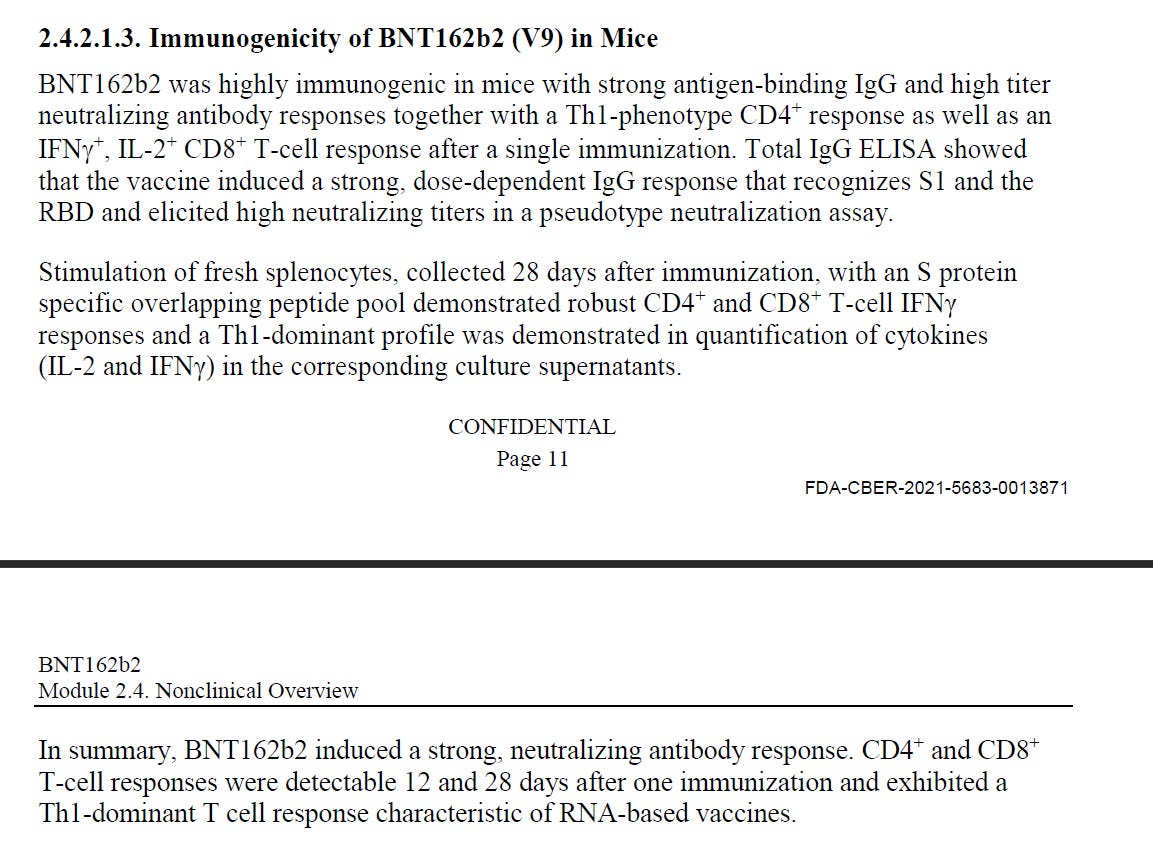
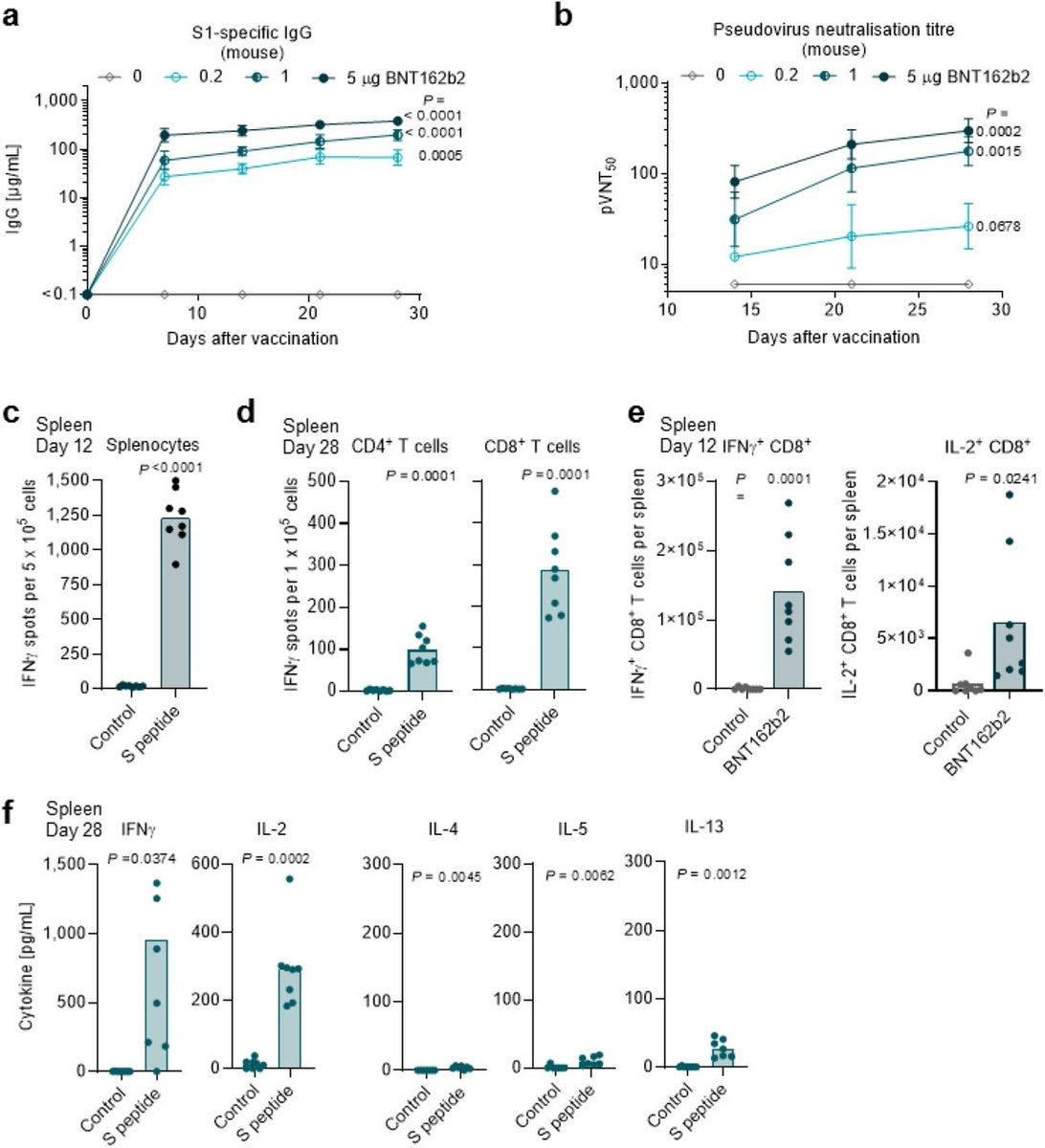
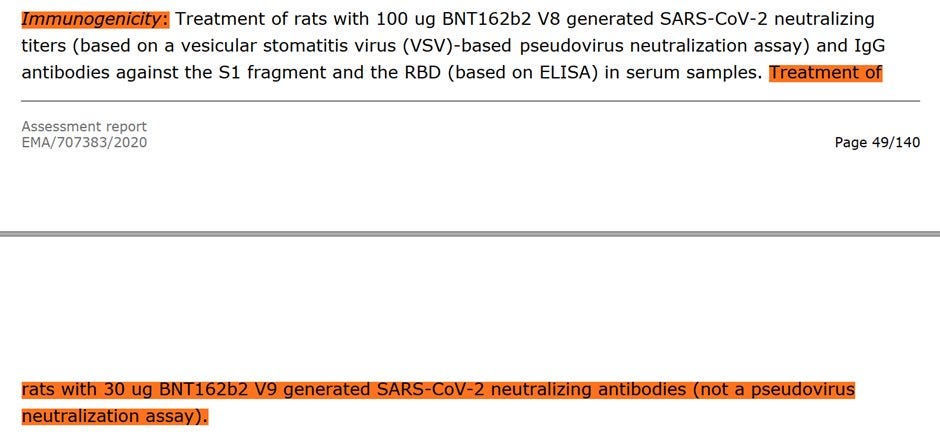
Mice: Pfizer/BioNTech injected 8 female mice with 5μg of BNT162b2 intramuscular (IM), and showed an IgG antibody response to the receptor-binding domain (RBD) and the S1. They showed immune response, not that their "vaccine" can protect from SARS-CoV-2.
The binding affinity
Pfizer/BioNTech stated that on day 28, the immunization showed IgG binding affinity of KD 12nM to the S1 OR 0.99nM binding affinity to the receptor-binding domain (RBD), or how well does the body's IGG attaches to parts of the spike (lower is better).
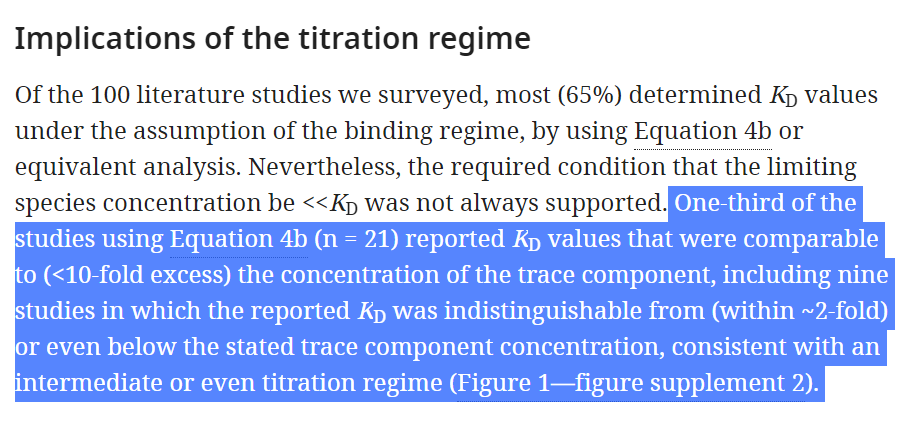
This Kd value depend on HOW Pfizer/BioNTech measured it. It is dependant on the concentration regime under which the reported Kd values were measured, and the time they waited to measure it! They can cheat by using a "Titration regime"!
"Titration regime" is when you have so much spike, that even with small immune response (IgG) will give a better Kd because there is higher chance of binding, which Pfizer/BioNTech could have used if they wanted to show high binding affinity, and didn't had one (source).
Pfizer/BioNTech stated a binding affinity of KD 12nM to the S1 vs 0.99nM binding affinity to the receptor-binding domain (RBD). It meant that their product was way LESS effective in binding to the S1 vs. the RBD. Now, why is it important? It's important because immune response (IgG) was much more likely to bind to the RBD of a SARS-CoV-2 than the spike itself, which CREATES NEUTRALIZATION ESCAPE VARIENTS, which Pfizer/BioNTech KNEW SINCE 2014! The S2 was MUCH better choice!
The Delta variant of SAR-SCoV-2 had 2 RBD mutations, and the omicron had 15 RBD mutation vs the original "wild type" Wuhan "virus". It greatly impacted the binding affinity (Kd), which Pfizer/BioNTech knew would happen, therefore they chosen to use the S1 ($$$)! Pfizer/BioNTech performed their assessment on Spleen and lymph nodes. They never assessed the lungs immune response, which is what I thought you suppose to prove if your product is supposed to stop infection and spread of a respiratory infectious disease.
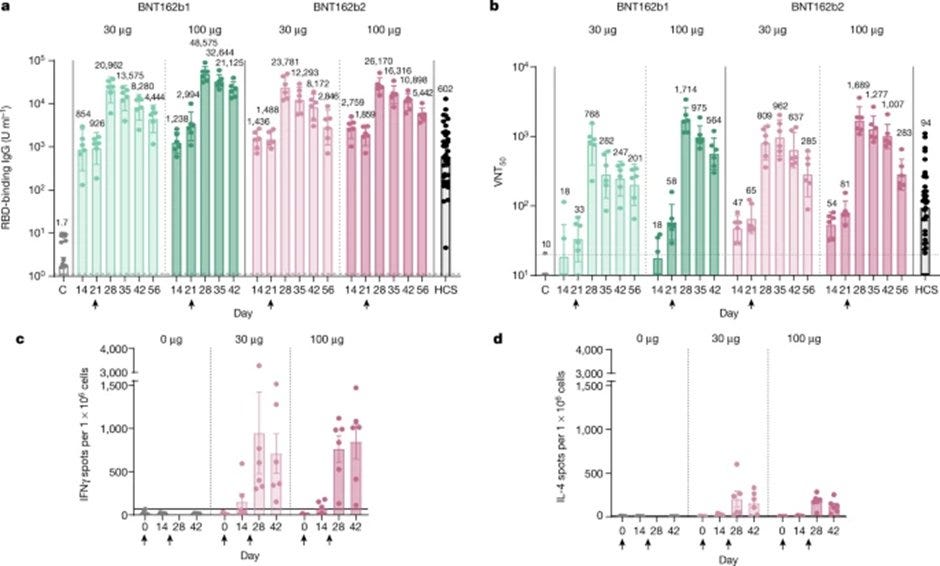
A small note - I will not cover Pfizer/BioNTech vitro experiment. I also decided to exclude the topic of the lipid nanoparticle because it is too technical for most people. Perhaps in another thread on the topic. Why? Because…Pfizer/BioNTech used Rhesus macaques monkeys in VR-VTR-10671, to prove immunogenicity. Pfizer/BioNTech claimed "at least an order of magnitude higher after BNT162b2 immunization of rhesus macaques than for the panel of SARS-CoV-2 convalescent human sera". Sound impressing. The “SARS-CoV-2 convalescent human sera (serum)" is SARS-CoV-2 neutralizing antibodies in sera from recovered COVID-19 patients. Here is article that describes the experiment, and below you can read what they got. I will cover this article into much deeper length in the next series, #MonkeyBusiness.
Does “more” means “better”?
Pfizer/BioNTech compared the geometric mean concentrations of RBD-binding IgG of the monkeys, 23,781 U ml−1 (30-μg dose level) for BNT162b2, to the RBD-binding IgG levels of a panel of 38 SARS-CoV-2-convalescent human serum, which was 602 U ml−1. Is more is better?
No. Having too many antibodies in your body is not a good thing, and Pfizer/BioNTech knew about it, as well as everyone who knows anything about the immune system. A value of 600 seems normal based on "SARS-CoV-2 Convalescent Sera Binding and Neutralizing Antibody Concentrations Compared with COVID-19 Vaccine Efficacy Estimates against Symptomatic Infection", Schuh et al, 2022:
It's worse! In mice, 5μg of BNT162b2 created geometric mean concentrations of RBD-binding IgG of 434,560. Pfizer/BioNTech decided that an immune concentration 40 times stronger in monkeys and 721 times stronger in mice then what humans had, is a good thing. Why?
Age Appropriate?
Last point - Pfizer/BioNTech used 2 to 4 year old monkeys. Macaques average age span is 25 years. This means they took, in human years, a 6 to 12 years old, and assumed it will work the same for adults with an immune system which very different in its capacities? Pfizer/BioNTech claimed their product (BNT162b) elicited protection in macaques, but they used the six monkeys that had been immunized using 100μg BNT162b2, not the 30μg (which was the dosage submitted to approval). Perhaps the protection was not good enough?
THE RATS
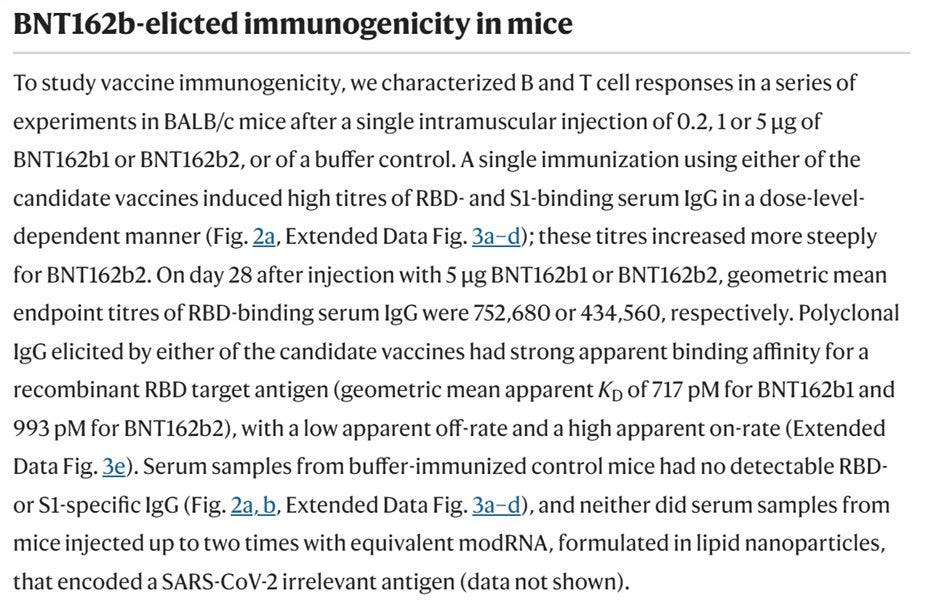
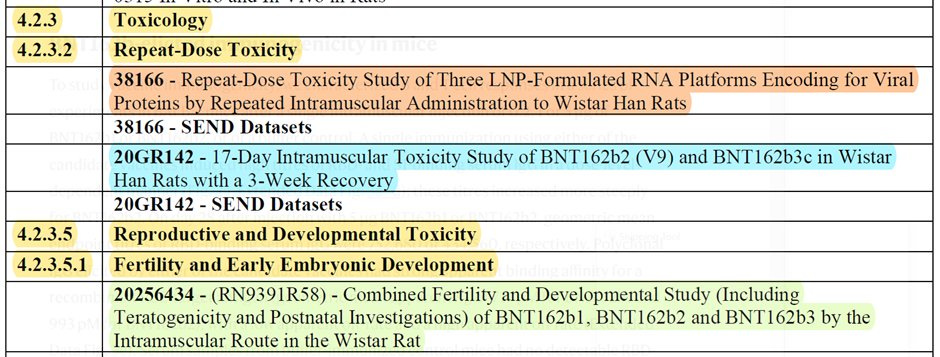
So finally, we come to the rats! Pfizer/BioNTech conducted 3 experiments on rats.
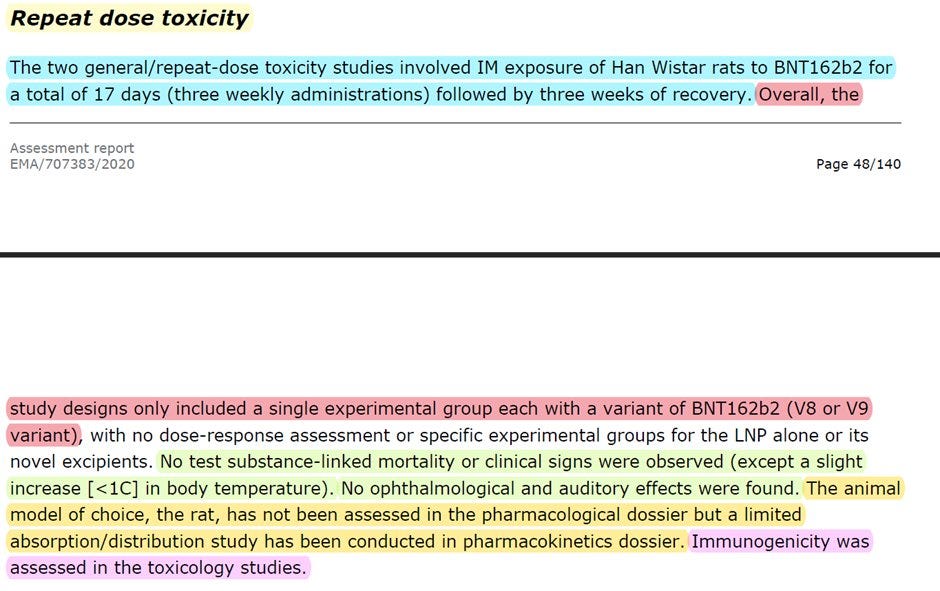
Pfizer/BioNTech conducted "two general/repeat-dose toxicity studies involved IM exposure of Han Wistar rats to BNT162b2 for a total of 17 days (three weekly administrations) followed by three weeks of recovery". 17 days does not seem like a lot, doesn't it? Well, because it is not. According to the creators of the "vaccine" (see above), Pfizer/BioNTech shortened the timeline from 3 weeks between each shot, the schedule for humans, to one week. I enclose the relevant paragraph from the book.
The repeat dosage toxicity was done using two formulas of Pfizer/BioNTech "vaccine", V8 and V9. V9 was the one approved, yet they still used the data from V8 as part of their submission. The difference? Codon Optimization (see #COptiGate). No one cared.
The redacted "INVESTIGATOR’S BROCHURE" (released via a FOIA) pages 37 to 50 gave more details about experiments 38166 & 20GR142.
Also, Sasha Latypova's review on the topic (which I only discovered after finishing my analysis) is here.
Last point,
There is the Pfizer/BioNTech "Combined Fertility and Developmental Study in the Wistar Rat GLP Study", which I will not go into it, because it is time to unveil the real secret.
THE SECRET
Remember the image from the beginning of the post? Allow me to remind you:
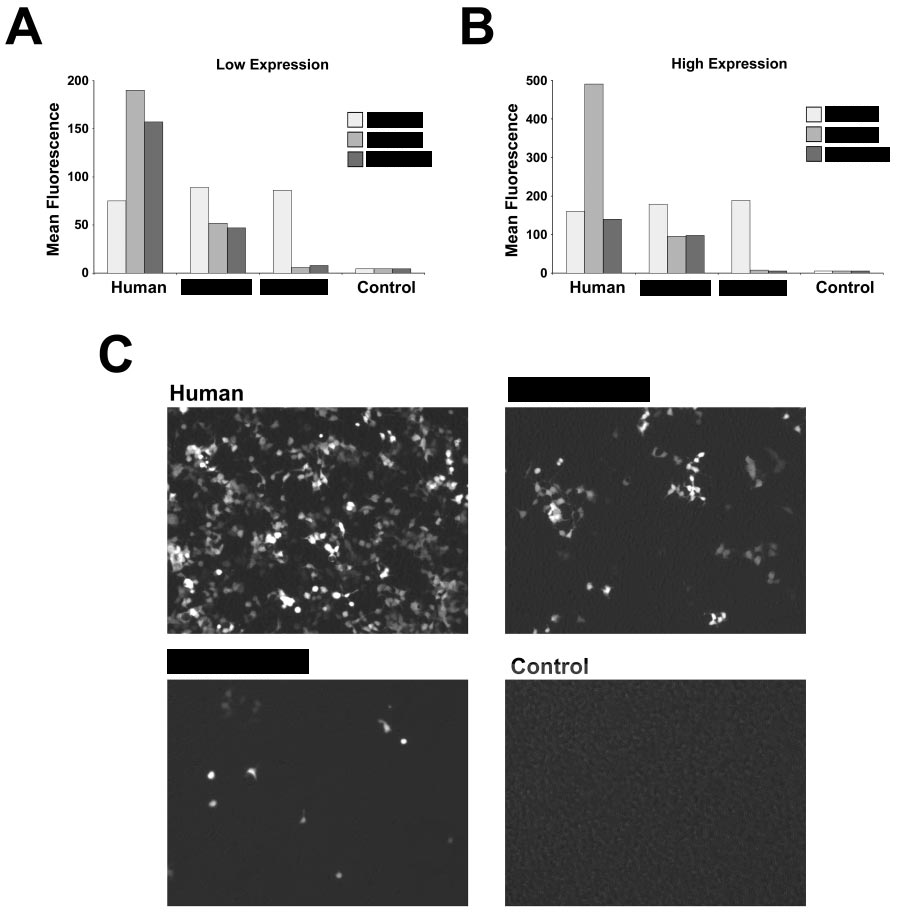
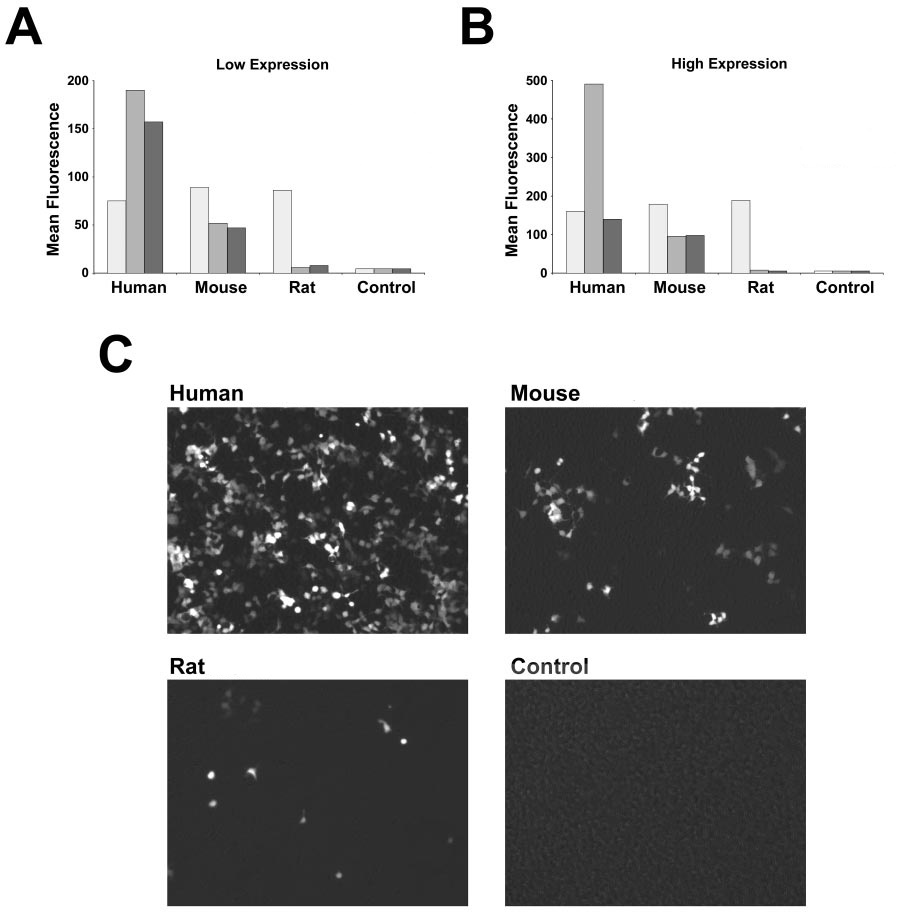
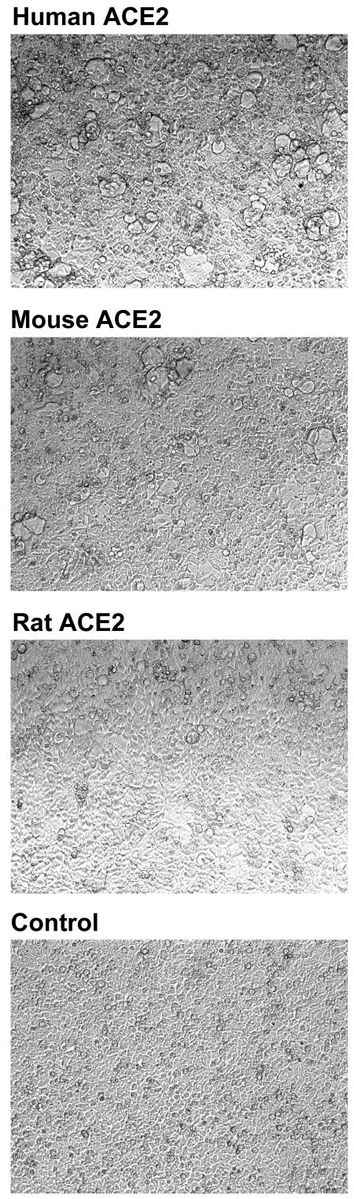
AS you can see below, the still-slightly-sanitised image describe differences between human, mice, and rats. It is obvious just by looking at the image that mice has much less expression and rats seems to barely have any expression of something. But what is it?
The answer is …ACE2!
ACE2
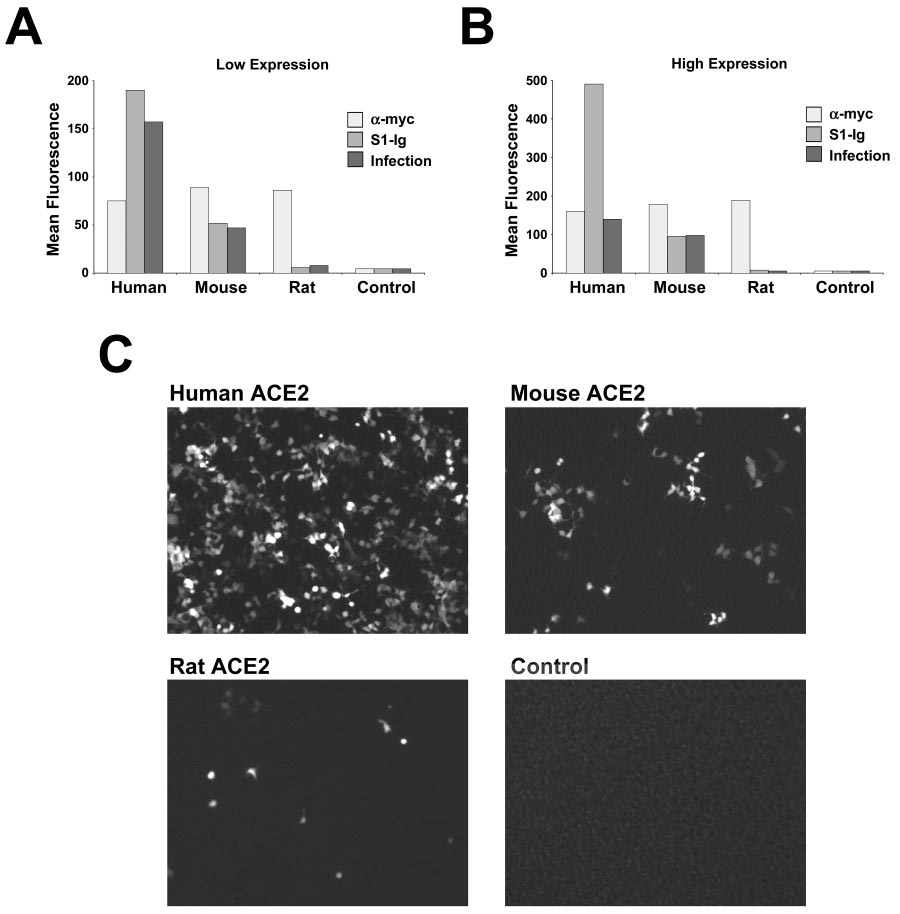
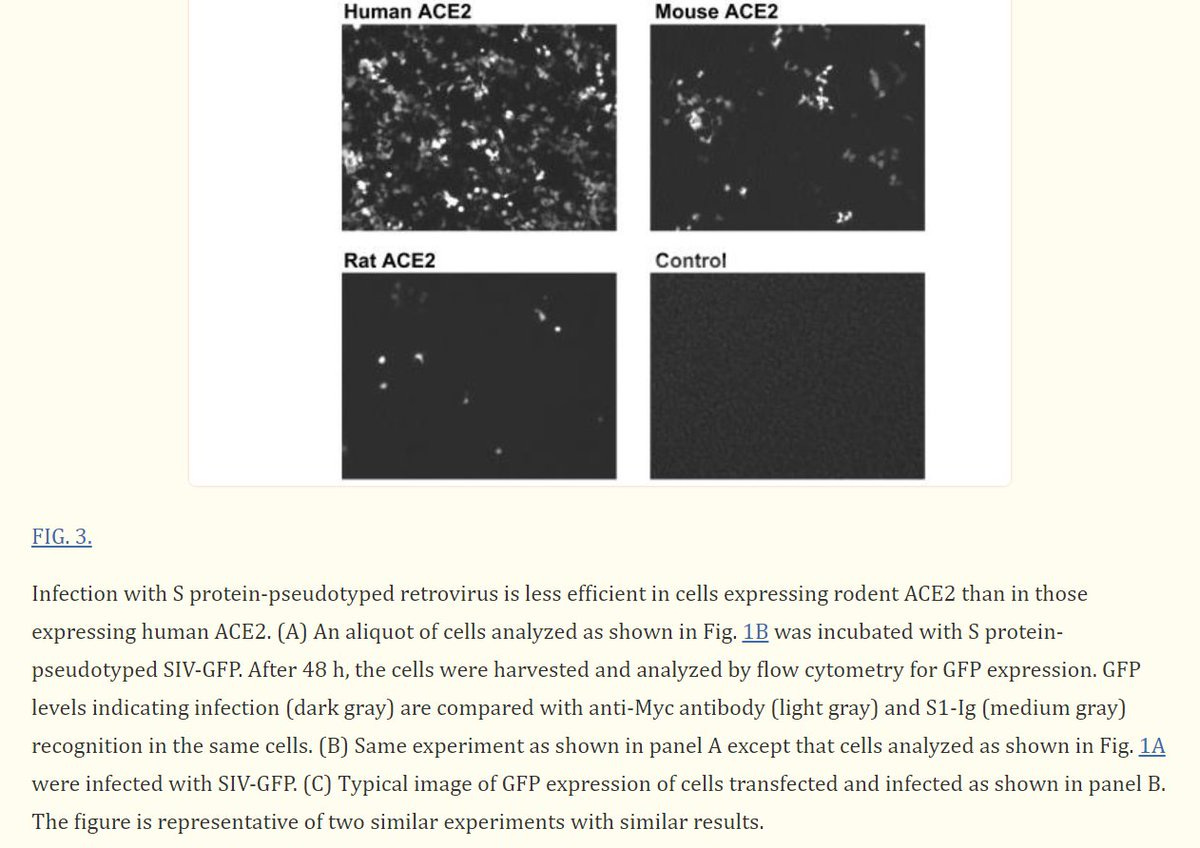
ACE2 is the receptor used by SARS-CoV-2 RBD, and as you can see the ACE2 receptors of humans, mice, and especially rats, responds VERY differently. Did Pfizer/BioNTech knew about it? As you will see in the next post, they did... SINCE 2004!
Scientists who studied SARS-CoV knew that "Infection with S protein-pseudotyped retrovirus (W/SARS-CoV S protein) is less efficient in cells expressing rodent ACE2 than in those expressing human ACE2".
I believe Pfizer/BioNTech, who wanted to get the emergency use authorization (EUA), has designed their experiments to use animals that they knew would have a very low chances of having severe adverse response to the spike protein created by their "vaccine".
Moderna Talking
To those who are still not certain that I know what I'm talking about when it comes to the ACE2 receptors, I give you…Moderna. Thanks, PanDanTag!
Pfizer didn't use animals that were fit for purpose because they own the regulators. That's why BioNTech went with them.
A criminal syndication.
VERO E6 CELLS
Pfizer/BioNTech also used Vero E6 cells to make the SARS-CoV-2 inoculum for the monkeys, but as was described in “SARS-coronavirus-2 replication in Vero E6 cells: replication kinetics, rapid adaptation and cytopathology Open Access”, which was published on June 2020 in the Journal of General virology, "compared to SARS-CoV, SARS-CoV-2 generated higher levels of intracellular viral RNA, but strikingly ABOUT 50-FOLD LESS INFECTIOUS VIRAL PROGENY".
THE RATS - THE SCAM
I BELIEVE PFIZER/BIONTECH DESIGNED THEIR EXPERIMENTS USING ANIMALS THAT WERE NOT FIT FOR PURPOSE.
Rat got injected with the "vaccine".
The lipid nanoparticles (LNP) penetrates the rat's cells. spike is being transcribed, translated and leave the cell.
The spike proteins barely binds to rats ACE2 receptors; the vast majority of them do not penetrate organs with ACE2 receptors (e.g. heart).
The rats body starts to create huge amount of spike-RBD-binding IgG, trying to get rid of the free spikes.
The "lonely" spike proteins (who don't have an ACE2 to bind to) can be problematic floating around (especially when it S2P structure is unstable, leading to disease prions), but it is better than the damage the spike is doing to the cells (e.g. mitochondria).
It is plausible Pfizer/BioNTech used lab animals that SARS-CoV-2 is barely able to infect or make sick, exposed them a lower infectious viral load, and used a form of "Titration regime", so they could "demonstrated" their product is "safe" and "effective".
DOSAGE: if my understanding is correct, the impact of what I described is that THE DOSAGES Pfizer/BioNTech SUBMITTED FOR APPROVAL ARE UNSAFE, and that their own tests results indicates that. If Pfizer/BioNTech made their calculations based on animal models that did not include ACE2 differences, the immune response they were looking for could only have been created using a high dosage of the "vaccine" to compensate the ACE2 differences. Think of a rat ACE2 as "underperforming". If Pfizer/BioNTech rely on ACE2, one approach will be to try to give a higher dosage of the product, to increase the chances of interaction in the rat body, to improve probability. The impact of such flooding (for the rat) is more activation of its immune system without a massive damage due to the spike->ACE2 "underperformance". The higher dosage that might work for the rat, but for someone else (human) it would overflow the ACE2 in their body.
There has been a lot of research & development of mice with a human ACE2. Here is one example. But #Pfizer/#BioNTech didn't had the time to get such animals, so, like with Codon Optimization (#COptiGate) they just didn't acknowledge it.
Regulatory Wordplay
In their submission to EMA Pfizer/BioNTech stated that "Rhesus macaques share a 100% homology with the human ACE2 sequence that interacts with the RBD of the S protein. BNT162b2". Homology = having common ancestor. It doesn't mean 100% match. It was a deception.
Pfizer/BioNTech only performed one biodistribution studied in rats (185350), and the limited it to the lipid nanoparticles. Rats ACE2 is so different that SARS-CoV infections were almost like a "background nose". Now that we know Pfizer/BioNTech failed to show safety or efficiency, EVERY ORGAN OF THE BODY that appeared in the biodistribution list is likely to have been harmed by the shots due to overdose, with higher risk to ACE2 enriched organs (e.g. heart).
Well tested technology?
Which bring me to another "fun fact":
Do you know how many people were treated with BioNTech experimental gene therapies, before Pfizer got into the COVID19 business with them? During 8 year… 400 cancer patients. ZERO background in treating infectious diseases.
Monkey Business - Prequel:
Here's a little prequel for the next thread: Do you know how many rounds of RT-qPCR Pfizer/BioNTech used to "identify" a #SARSCoV2 positive animal? 45 cycles !!! If Kary Mullis was alive he would have destroyed these "scientists". These were not scientific experiments, but a hoax.
Thank you for reading…
Ehden Biber



















EVERYONE!! Go to brighteon.com and type in “WHO whistle blower exposes global agenda “it’s an interview with del big tree, and it is powerful
Thank you for yet another superbly researched article!