THE LONG TERM SAFETY STUDY
Why did AZ asked to withdraw their EUA marketing authorisation approval, and how come you didn't hear about it? THIS IS AN UPDATED VERSION!!!
UPDATE - THE NEXT ARTICLE WILL CORRECT EVERYTHING WHICH WAS INCORRECT IN THIS ARTICLE (far beyond what I’ve spoken about with
on his podcast). It’s going to be A LONG article, and after I will publish it, this article should be considered as VOID. The only reason I didn’t amend it at all is because I wanted to show people that the process of investigating such a situation means we make mistakes. And no, it doesn’t mean that AZ study showed safety. The opposite, it’s… well…WAIT FOR IT.
Thank you for your understanding
Ehden
As you might have heard, AstraZeneca ASKED in March 2024 to have their COV!D19 SH0TS product (ChAdOx1 / Vaxzevria / AZD1222) approval withdrawn from Europe's EMA. It was not the European Union (EMA) who decided to withdraw their approval due to the damage they caused people. It will become effective on the 7th of May 2020. BUT WHY DID THEY DO IT?
To understand what happened, let's start with a QUICK, SHORT reminder before we will go into the details.
JANUARY 2021: EMA recommends COVID-19 Vaccine AstraZeneca for authorisation in the EU
11 March 2021:EME's Pharmacovigilance Risk Assessment Committee (PRAC) investigated cases of thromboembolic events, but claimed the vaccine’s benefits currently still outweigh risks. Denmark, Norway, and Iceland suspended use of the product.
31 MARCH 2021: Many EU countries resumed the use of AZ products, but restricted it to people above certain age.
BACK TO THE STUDY. The EMA has requested AZ to perform multiple risk assessments of their products. It is all mentioned here. One of them was the clinical trial D8110C00001 : "A Phase III Randomized, Double-blind, Placebo-controlled Multicenter Study in Adults to Determine the Safety, Efficacy, and Immunogenicity of AZD1222, a Non-replicating ChAdOx1 Vector Vaccine, for the Prevention of COVID-19"
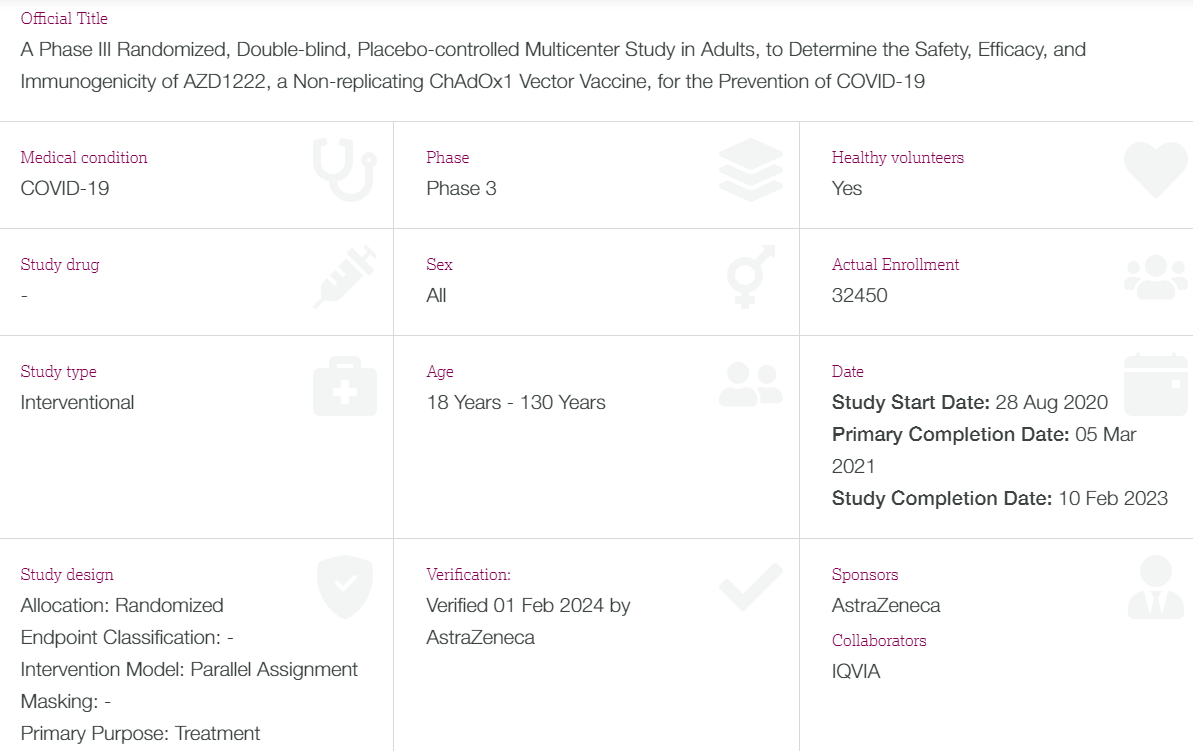
The study started on 28 Aug 2020, it's primary completion date was supposed to be 05 Mar 2021, reported to the EU Clinical Trials Register as completed on 21 Mar 2023 and submitted 23 Nov 2023.
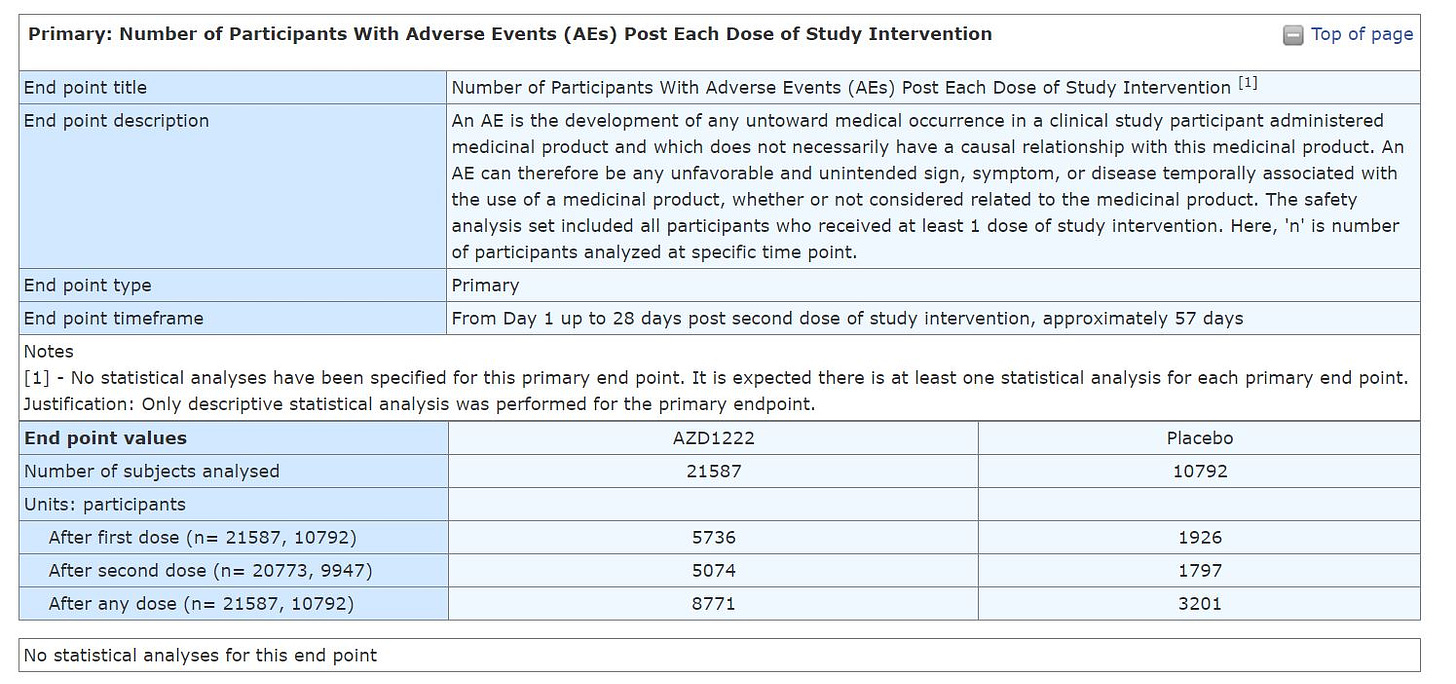
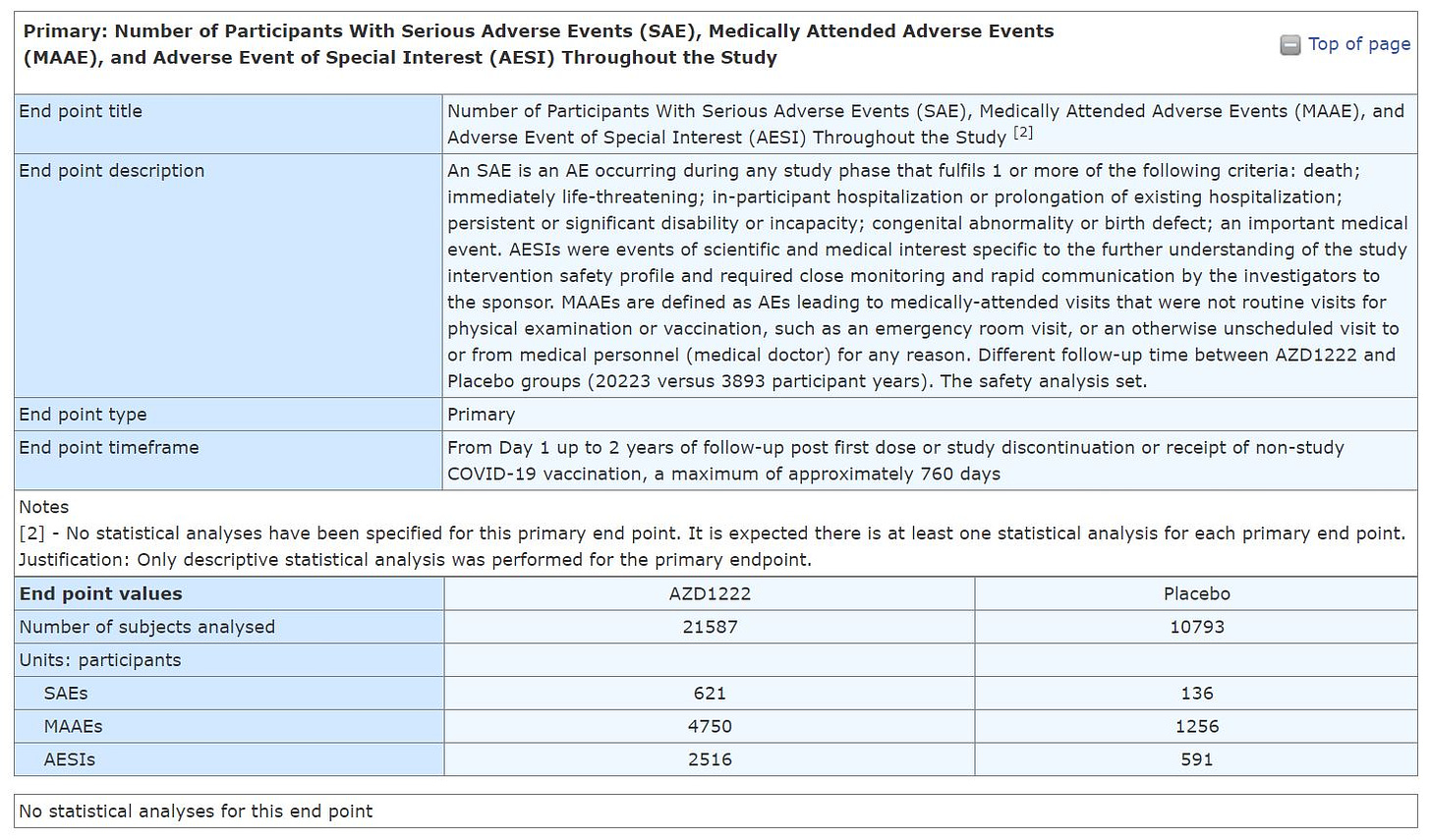
Notice that the placebo group is about 50% in size of the group that received the product AZ (AZD1222). This means that any Adverse Events (AEs), Serious Adverse Events (SAE), Medically Attended Adverse Events (MAAE), and Adverse Event of Special Interest (AESI) Throughout the Study should have had half of the # of events in the group that received the product.
IT DIDN'T.
Why are there any adverse events with the placebo? Because in September 2021 most people in the placebo and the study arm received boosters, so even though up to September 2021 they had a group that received saline placebo, from that moment on they
Adverse Event of Special Interest (AESI) group is defined by the researchers, which means that events such as myocarditis which normally be described as SAEs could be defined as AESIs, which allows the researchers to claimed they have a low amount of Serious Adverse Events.
Notice the statement: "No statistical analyses have been specified for this primary end point. It is expected there is at least one statistical analysis for each primary end point.
Justification: Only descriptive statistical analysis was performed for the primary endpoint."
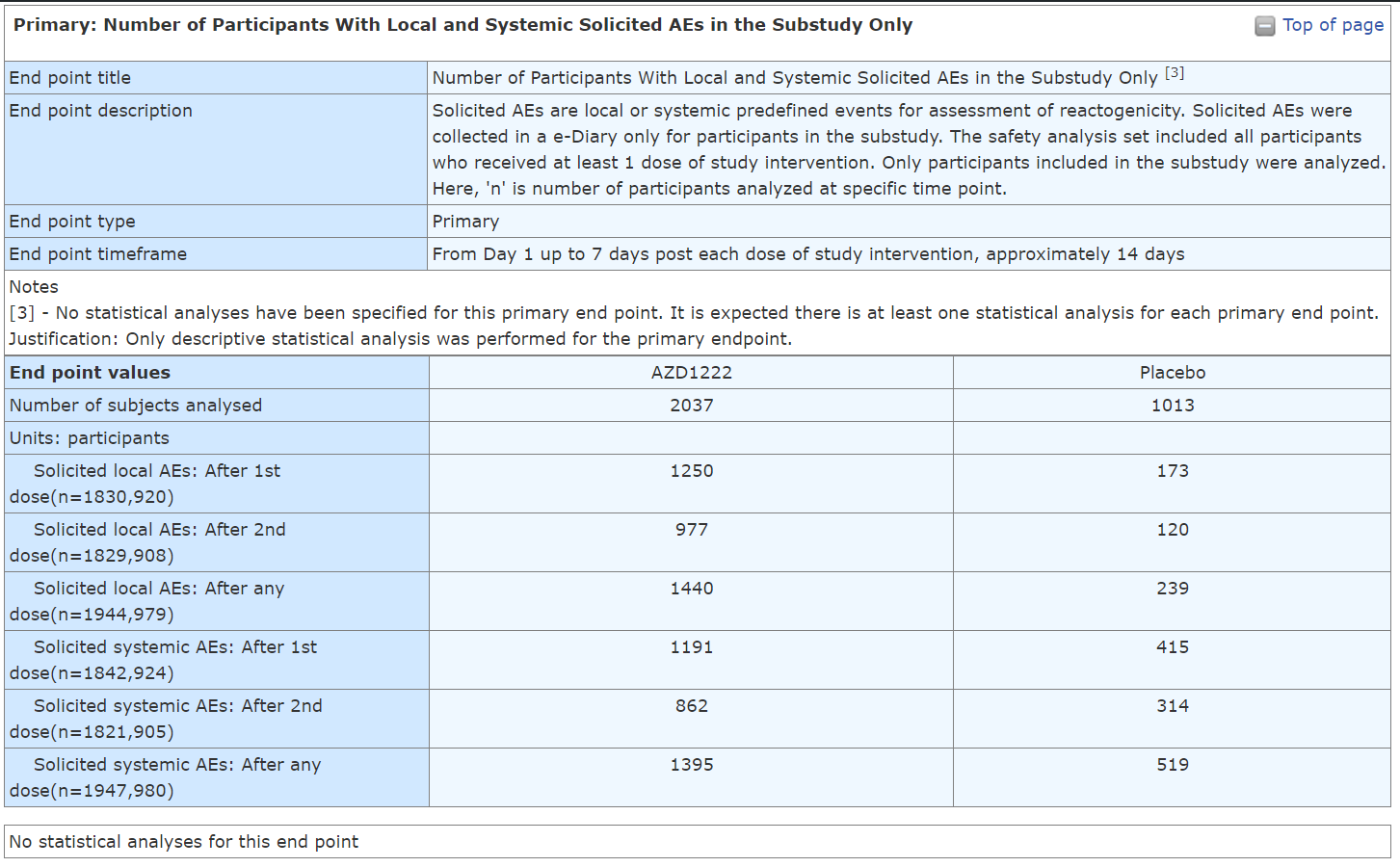
PS - There was another substudy, which look at the number of Participants With Local and Systemic Solicited AEs. If I'll find more information I'll add. Here are its results:
YOU DO NOT NEED TO BE A STATISTICIAN TO SEE THAT THE EVENTS IN THOSE WHO RECEIVED THE AZ COV!D19 SH0TS GROUP ARE WAY MORE THAN DOUBLE THAN IN THE PLACEBO.
I'll let statisticians do the math...
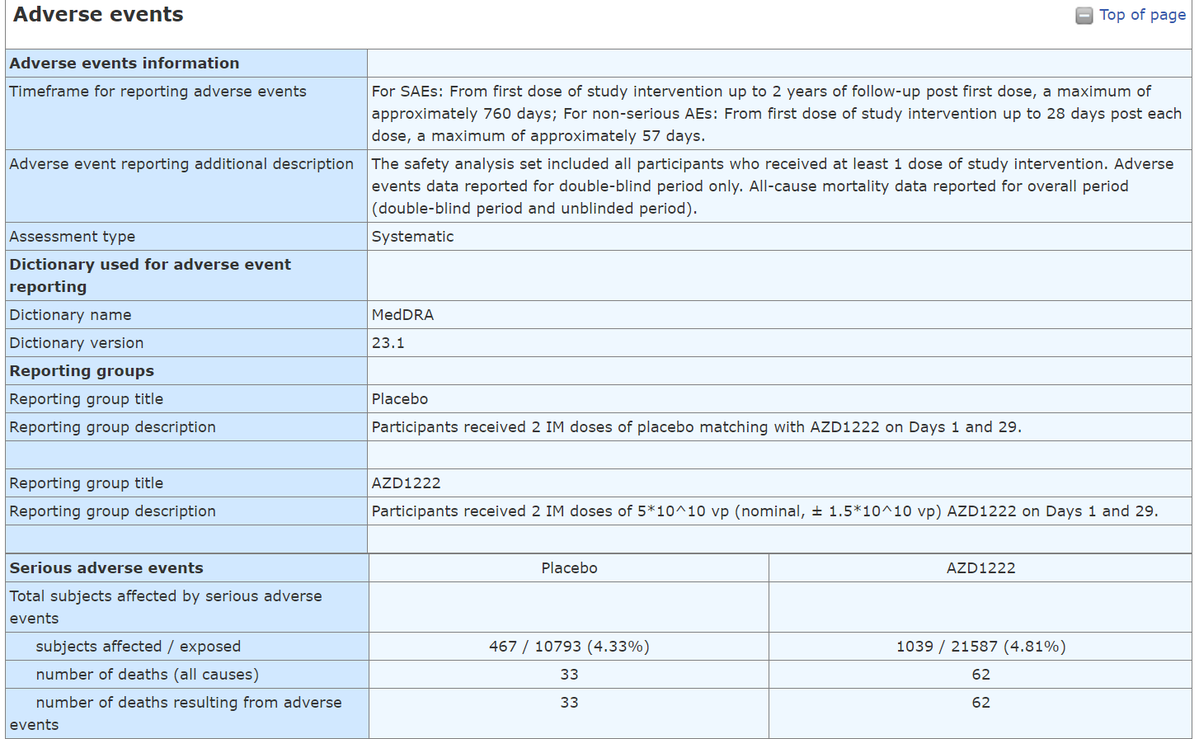
Now look at the "Adverse Events" table, which AFAIK was the one that was reported to the regulator. It left me puzzled as it seems to be completely not in line with the results I've mentioned above. Why the discrepancy? We can't tell with no analysis SAEs, MAAEs, and AESIs.
This study was reported on the Committee for medicinal products for human use (CHMP) meeting that took place between 18-21 March 2024, with "Positive Opinion adopted by consensus on 07.03.2024."
In the same meeting, it was announced that AZ has applied for the withdrawal of their "Vaxzevria - COVID 19 Vaccine (ChAdOx1 S [recombinant])" marketing authorisation. Why? IMHO the endpoints of SAEs, MAAEs, and AESIs and their lack of analysis clearly showed the reason.
The timeline of this research and its results indicates why AstraZeneca has decided to apply for the withdrawal of their marketing authorisation.
Next time someone tells you that something is "Safe and effective", remind them this study that showed no long term safety.
Do we know what is the EMA position on the safety of this product NOW THAT THEY GOT THE LONG TERM SAFETY STUDY RESULTS? Oh wait, they don’t need to tell us. The product has been withdrawn so no need to look further. How continent for AstraZeneca and the EMA.
Simply unbelievable!




Regarding the use of placebos in vaccine trials, the book *Turtles All The Way Down* is a must read - absolutely shocking!
Hi Ehden, the 'placebo' used in the AZ trials was the meningitis vaccine. https://www.europarl.europa.eu/doceo/document/P-9-2020-005248_EN.html
It's highly unusual for drug companies to use saline (which Pfizer allegedly did in their covid vax trial) so there are virtually no double-blind placebo-controlled trials for any vaccine. All results are skewed because of this.