(originally posted on my twitter account on the 1st of October 2021)
Why today's announcement that #molnupiravir cuts risk of #COVID19 hospitalization and death in half IS NOT GOOD NEWS, but a demonstration of #BigPharma murky business, or in the case of #Merck, #MerckyBusiness.
If you believe, for example, @DrEricDing, a senior fellow of the Federation of American Scientists, it is time to open our Champagne bottles. An antiviral drug can cut hospitalization and death in half in a randomized trial. Finally!
It is such great news that the U.S. government already made an advance purchase of 1.7 million doses of the drug at a cost of $1.2 billion.
Great news, we are being told.
LET US LOOK AT THE FACTS.
#molnupiravir (AKA MK-4482/EIDD-2801) is not really a new drug, in the sense that it is a prodrug of N4-hydroxycytidine (NHC), which means it was designed to improve the bioavailability of how it absorb, distributed, metabolized, and excreted.
"N4-hydroxycytidine was first described in the literature in 1980 as a potent mutagen of bacteria and phage".
N4-Hydroxycytidine, N(4)-Hydroxycytidine, Beta-D-N4-hydroxycytidine, and EIDD-1931 are all synonyms.
pubchem.ncbi.nlm.nih.gov/compound/N_4_-…
N(4)-Hydroxycytidinehttps://pubchem.ncbi.nlm.nih.gov/compound/N_4_-Hydroxycytidine
We now know that Molnupiravir works by promoting SARS-CoV-2 mutagenesis, which means that this drug promotes the genetic information of an SARS_CoV_2 to change by the production of a mutation, and by doing so is supposed to disable it.
Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA templateThe RNA-dependent RNA polymerase of the severe acute respiratory syndrome coronavirus 2 is an important target in current drug development efforts for the treatment of coronavirus disease 2019. Molnup…https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8110631/
But what also do we know about the compound which this product is based upon?
Well, quite a number of alarming things.
N4-Hydroxycytidine is TOXIC:
N4-Hydroxycytidine (NHC) "exhibits measurable levels of cytotoxicity, with 50% cytotoxic concentration values … in cell lines"
Analysis of the Potential for N 4-Hydroxycytidine To Inhibit Mitochondrial Replication and Function - PubMedN 4-Hydroxycytidine (NHC) is an antiviral ribonucleoside analog that acts as a competitive alternative substrate for virally encoded RNA-dependent RNA polymerases. It exhibits …
"β-D-N4-hydroxycytidine (rNHC) and its orally bioavailable prodrug, molnupiravir, does inhibits SARS-CoV-2 in vitro BUT IS MUTAGENIC IN MAMALIAN CELLS…resulting in DNA mutation of dividing mammalian cells."
https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1250916
And also, based on whistleblower charges of cronyism behind the drug, that "some earlier studies suggested EIDD-2801 (#molnupiravir) COULD CAUSE HARMFUL GENETIC MUTATIONS".
Emails Offer Look Into Whistleblower Charges of Cronyism Behind Potential COVID-19 DrugEarly last month, a top contracting officer at a U.S. agency charged with accelerating the development of drugs to fight the COVID-19 pandemic got a request that made him feel uneasy. Senior
So quick recap - a drug that can cause DNA damage, AND which is a bioavailable version what exhibits measurable levels of 50% toxicity to cells is considered to be good news that #Merck is asking for emergency authorization FOR THE WORLD?
How by looking at 775 adults who had health problems with mild-to-moderate COVID-19, and with a period of trial of 30 days which resulted in 6.8% reduction in hospitalization or death (14.1% vs 7.3%) Merck wants such authorization?
How come @DrEricDing, an Epidemiologist & health economist, Senior Fellow @FAScientists, w/former 16 years @Harvard, a @JohnsHopkins alum is reporting on this drug as if we found the fountain of life?
Doesn't he care about the dangers of this drug?
I want to remind everyone that #Merck already in the past has suppressed the clinical trial evidence about the dangers of one product, #VIOXX, that led to the death of many people.
Merck Vioxx scandal"We expect accountability, we expect them to be open with us, we expect them to be honest with us." That comment was made last week by Marsha Robbins, the forewoman of the jury that found Merck liabl…
The fact a medical product has one desirable effect does not allow us to ignore the other impact of that product on the body. Good example is radiation - it can kill cancer cells, but also is deadly to your body.
Early victims of X-rays: a tribute and current perceptionX-rays were discovered in 1895 and since then much has been written about Wilhelm Roentgen and the events surrounding the discovery. However, there have been only scattered references in the literatur…
A drug that carries zero liability under emergency authorization = goldmine for Merck.
This drug might lead to DNA damage and cytotoxicity yet "scientists", media & regulators don't care. We are in a post-modernism era: science is dead, long live scientism!
Take a close look at who is hailing this drug (media, "scientists", "journalists"), and realize that THEY DO NOT CARE ABOUT YOUR HEALTH, THEY DO NOT CARE ABOUT YOU.
Notice how these are the same people who have been "selling" you facemasks, lockdowns & vaccines.
"similar experimental drugs in this class had been shown to cause reproductive toxicity in animals, and OFFSPRING FROM TREATED ANIMALS HAD BEEN BORN WITHOUT TEETH AND WITHOUT PARTS OF THEIR SKULLS"
PLEASE READ HERE:
pulitzercenter.org/stories/emails…
Thank you @TigerBosco!
Emails Offer Look Into Whistleblower Charges of Cronyism Behind Potential COVID-19 DrugEarly last month, a top contracting officer at a U.S. agency charged with accelerating the development of drugs to fight the COVID-19 pandemic got a request that made him feel uneasy. Senior Trump...https://pulitzercenter.org/stories/emails-offer-look-whistleblower-charges-cronyism-behind-potential-covid-19-drug
Read the article (Pulitzer Center), and ask yourself - how can a bioavailable version of what has such bad safety history suddenly being "sold" as a miracle drug?
Why is the whistleblower testimony (safety, cronyism) suddenly not relevant?
#MERCKyBusiness
Emails Offer Look Into Whistleblower Charges of Cronyism Behind Potential COVID-19 Drug
Early last month, a top contracting officer at a U.S. agency charged with accelerating the development of drugs to fight the COVID-19 pandemic got a request that made him feel uneasy. Senior Trump...
To the claims that genotoxicity was disproven …
Wait for it 😉
@Millinillion3K3 @omerrulu @US_FDA I've waited for the "it was disproven!" claims, thank you! hahaha Yes - Merck done tests to disprove the genotoxicity claims … on which I will expand on my next update of this thread, in which I will show how Merck is the one guilty in a 'lie of omission' 😉 #MERCKyBusiness
#MERCKyBusiness - disproved² thread
In this part I will address the claims that Merck has already addressed the genotoxicity concerns, because obviously, the minute you make a claim against #BigPharma you are assured to get "fact checkers" demanding to cancel you.
👇👇👇
The whole saga started with a research I've mentioned its results above, entitled "β-D-N 4-hydroxycytidine (NHC) inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells.".
https://doi.org/10.1093/infdis/jiab247
Zhou et al claimed that "rNHC (or molnupiravir) Is Mutagenic in a Mammalian Cell Assay" and that "there are risks for the host in that the same mutagenic activity that impacts viral replication has the potential for incorporation and mutagenesis of host DNA."
They also stated that "mutations in host DNA COULD CONTRIBUTE TO THE DEVELOPMENT OF CANCER, , OR CAUSE BIRTH DEFECTS EITHER IN A DEVELOPING FETUS OR THROUGH INCORPORATION INTO SPERM PRECURSOR CELLS".
What was even more worrying is that a short therapy would not prevent the host from exposure "because both RNA precursors that affect the virus and DNA precursors that would affect the host pass through the common ribonucleoside diphosphate intermediate."
This started disproved² saga. Let us look at "Developing a direct acting, orally available antiviral agent in a pandemic: the evolution of molnupiravir as a potential treatment for COVID-19" ("Current Opinion in Virology", Oct 2021)
https://doi.org/10.1016/j.coviro.2021.06.003
This article (AFAIU) is an editorial by the researchers who developed/worked on molnupiravir, telling the story of their work. It is useful to start with it, even though it is retrospective, as it gives good context.
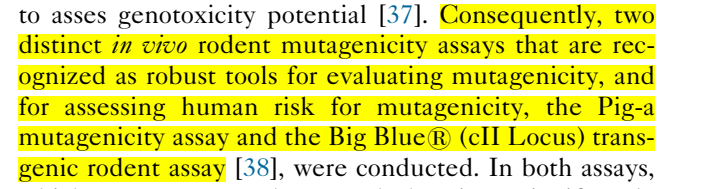
First, the predecessor of molnupiravir (EIDD-2801) was EIDD-1931, which has the capacity to cross the blood–brain barrier, as it was developed to treat VEEV (Venezuelan equine encephalitis virus).
AFAIU, It means molnupiravir can cross the brain barrier.
"EIDD-1931 was orally bioavailable, widely distributed to organs including the lungs and appeared to be actively transported into the CNS where it was quickly anabolized to the active 5'-triphosphate"
EIDD-1931 inhibits replication of multiple RNA viruses of influenza, various coronaviruses, respiratory syncytial virus (RSV), VEEV), Chikungunya and Ebola (in animal models). HOWEVER, it metabolized quickly in non-human primates.
EIDD-2801 (molnupiravir) is a prodrug of EIDD-1931, which "facilitated movement across the gut lining and EFFICIENTLY DELIVERED EIDD-1931 to the circulating volume of all species tested, including non-human primates"
*BREAK*
Notice - molnupiravir is in fact a drug that was designed to deliver EIDD-1931 in an efficient way.
Now comes the interested section - the genotoxicity tests:
"Because of a positive Ames test, the potential for genotoxicity has been thoroughly evaluated for molnupiravir both in vitro and in vivo."
Ames test use a bacteria to test if a given chemical can cause mutations in the DNA of an organism being tested.
(JUST A REMINDR)
In vitro ("in the glass" in Latin) - test performed on living tissue.
In vivo ("in the living" in Latin) - test on an organism, such as a rodent, or human beings.
According to the article, they performed two
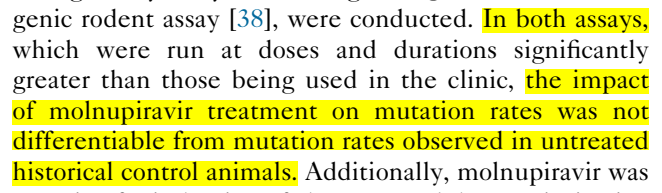
in vivo rodent mutagenicity assays: the Pig-a
mutagenicity assay, and the Big Blue1 (cII Locus) transgenic rodent assay.
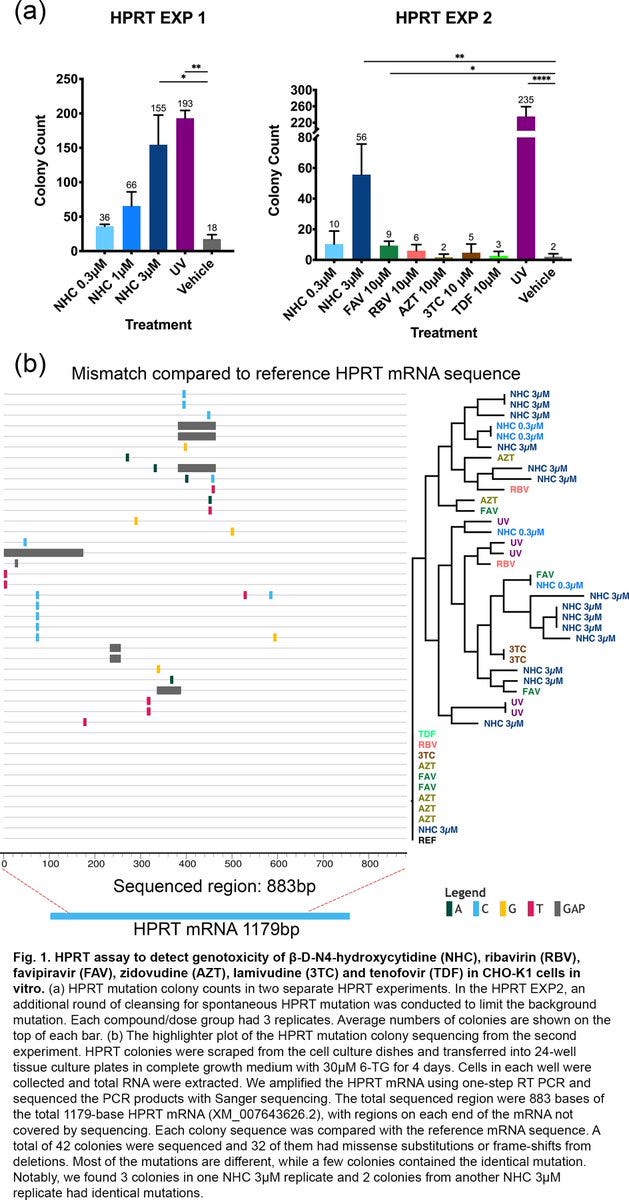
According to the authors, "in both assays…the impact of molnupiravir treatment on mutation rates was not differentiable from mutation rates observed in untreated historical control animals."
Even better, "molnupiravir was negative for induction of chromosomal damage in in vitro micronucleus (with and without metabolic activation) and in vivo rat micronucleus assays."
Which led the authors to conclude "based on the totality of genotoxicity data molnupiravir is not considered to pose an increased risk of genotoxicity in clinical use."
Problem solved, right?
Not so quick.
Let's dig in a little bit.
IN OUR UPCOMING SECTION, I will look at a more detailed of the actual tests, ask if it was really disproved, and … well, perhaps even reach the disproved² phase.
Digging deeper into the saga.
To learn more about what was actually done, let's head to "Letter to the Editor in Response to Zhou et al" ("The Journal of Infectious Diseases", Troth et al., Jul 2021)
https://doi.org/10.1093/infdis/jiab362
This letter describes the actual disprove activities that were done as a response to the letter I've mentioned above.
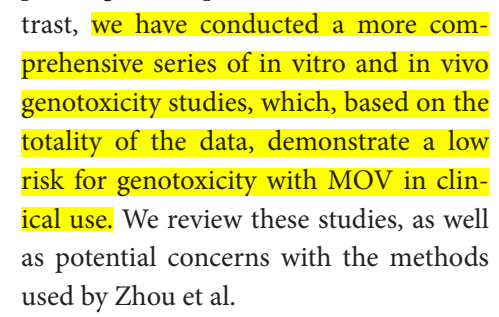
As mentioned above, the authors state that they "have conducted a more comprehensive series of in vitro and in vivo genotoxicity studies, which, based on the totality of the data, demonstrate a low risk for genotoxicity with MOV in clinical use."
The authors confess/agree that rNHC (or molnupiravir) is mutagenic in vitro, but claim that this is not relevance in vivo.
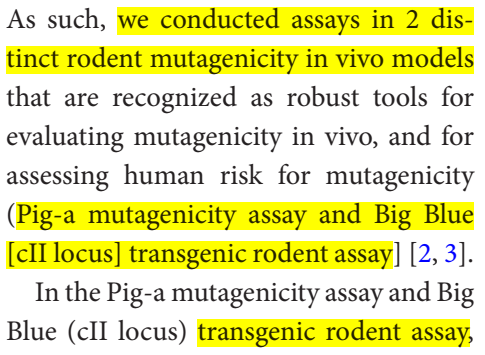
And, as was mentioned above, they conducted 2 experiments in 2 distinct rodent mutagenicity in vivo models. (Pig-a mutagenicity assay and Big Blue [cII locus] transgenic rodent assay.
According to the authors, "The impact of MOV (molnupiravir) treatment on mutation rates was not differentiable from mutation rates observed in untreated historical control animals."
Let's zoom in.
The two experiments referenced have not been published nor peer reviewed, and were conducted on rats.
Pig-a was done by Charles River Laboratories
https://www.criver.com/research-phase/clinical-development
And Big Blue was done by BioReliance:
bioreliance.com/us
The scientists used Pig-a assay. Let's learn more about this method, from "The Pig-a Gene Mutation Assay in Mice and Human Cells: A Review" (Olsen et al, 2017).
The Pig‐a Gene Mutation Assay in Mice and Human Cells: A ReviewThis MiniReview describes the principle of mutation assays based on the endogenous Pig-a gene and summarizes results for two species of toxicological interest, mice and human beings. The work summari.…
Even though the work summarized in this paper largely avoids rat-based studies, it does raise important points.
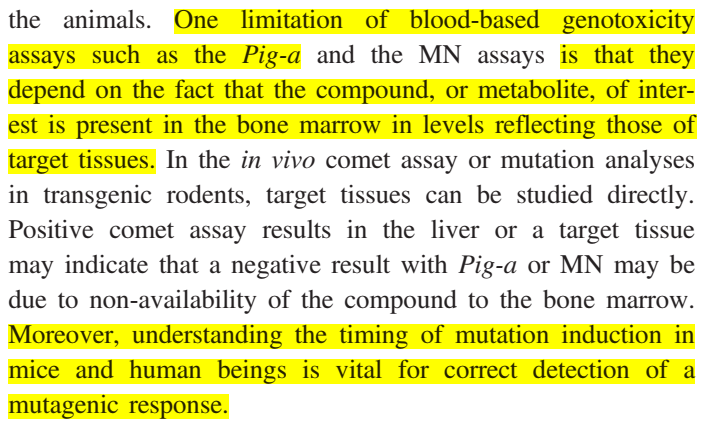
Pig-a uses only minute blood volumes, so it is a fast test (rather than killing the rat and doing an analysis of the organs.
HOWEVER, this test "depend on the fact that the compound, or metabolite, of interest is present in the bone marrow in levels reflecting those of target tissues…moreover, understanding the timing of mutation induction…is vital for correct detection"
Another disadvantage to the pig-a method of mutagenic investigation is that unlike alkaline comet assay it does not detect pre-mutagenic DNA lesions.
(Chromosomal aberrations in the form of broken fragments of chromosomes can lead to pre-mutagenic lesions).
The researchers did look at chromosomal
damage in micronucleus assays
"The advantages and disadvantages of the cytokinesis-blockmicronucleus method" describes the limitation of this investigation:
https://doi.org/10.1016/S0165-1218(97)00041-4
"This method does not distinguish between dividing and non-dividing cells … it is unable to provide a measure of more subtle changes, such as balanced translocations."
"IT IS, therefore, WRONG TO ASSUME THAT A MICRONUCLEUS ASSAY CAN REPLACE THE DETAILED ANALYSIS OF CHROMOSOME DAMAGE AFFORDED BY METAPHASE ANALYSIS"
More limitations: "Perhaps the major concern with the use of (this method) is that its use may prevent the detection of chemicals that are also inhibitors of cytokinesis or microfilament polymerisation."
"The prospect of using long-term cultured human lymphocytes in in-vitro testing is likely to be more relevant in predicting human risk than using non-human cell".
Which brings us back to molnupiravir and the Big Blue [cII locus] transgenic rodent assay.
Remember I've mentioned pig-a does not identify pre-mutagenic DNA lesions? The other test that was done was a transgenic rodent mutation assay.
Limitation of the method:
"The transgenic mice models respond to mutagens in a similar manner to endogenous genes and are suitable for the detection of point mutations, insertions and small deletions but not large deletions"
https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2011.2379
Also, Transgenic Rodent (TGR) mutation assays does not seems to detect pre-mutagenic lesions - at least it is my understanding from the wording of this OECD document.
If I'm wrong, please correct me.
https://doi.org/10.1787/20745788
OECD Guidelines for the Testing of Chemicals, Section 4The OECD Guidelines for the Testing of Chemicals is a collection of testing methods to identify and characterise potential hazards of new and existing chemical substances. This group of tests covers h…
I believe that the claims that genotoxicity has been disproved are weak, due to the limitations of the methods used, plus the lack of visibility to the details of the in vivo mutagenicity tests and the in vitro micronucleus chromosomal damage tests.
I will look into more claims the molnupiravir researchers made to disprove the genotoxicity claims, and the response that came as a result …
In this segment I am going to continue and disprove the claims of disprove the researchers behind molnupiravir has made, or in other words - did the researchers proved it is safe?
First of all, a FAST recap:
After Zhou et al has suggested that molnupiravir is neurotoxic, Troth et al replied claiming the tests they conducted showed the product is not neurotoxic. They have done 2 in vivo tests, one using Pig-a.
https://doi.org/10.1093/infdis/jiab362
A possible explanation why they decided on pig-a can be found in the pre-print ed. of "Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity against SARS-CoV-2".
Human Safety, Tolerability, and Pharmacokinetics of a Novel Broad-Spectrum Oral Antiviral Compound, Molnupiravir, with Activity Against SARS-CoV-2Molnupiravir, EIDD-2801/MK-4482, the prodrug of the ribonucleoside analog ß-d-N4-hydroxycytidine (NHC), has activity against a number of RNA viruses including severe acute respiratory syndrome coronav…
44/x
In it, we find out that "the dose-limiting toxicity in one Investigational New Drug-enabling study … was bone marrow toxicity and included reversible reductions in platelet counts" (this statement disappeared in the final edition).
https://doi.org/10.1128/AAC.02428-20
Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity against SARS-CoV-2 | Antimicrobial Agents and ChemotherapyMolnupiravir (EIDD-2801/MK-4482), the prodrug of the active antiviral ribonucleoside analog β-d-N4-hydroxycytidine (NHC; EIDD-1931), has activity against a number of RNA viruses, including severe acut…
Pig-a is supposed to be good in detecting bone-marrow related neurotoxicity.
However, I've already written about the limitations of the Pig-a test:
The molnupiravir Pig-a tested peripheral red blood cells, RBCs (according to Troth et al).
HOWEVER, measuring Pig-a gene mutation in RBCs seems to be less accurate than measuring gene mutations in reticulocytes (RET) (immune red blood cells).
In a paper published on the topic, out of 24 chemicals, only 3 produced positive response in RBCs vs 13 in RET, and only 3 showed (after a week) Pig-a mutant frequency in RBC vs 12 in RET.
https://www.sciencedirect.com/science/article/pii/S1383571816303291
The PIGRET assay, a method for measuring Pig-a gene mutation in reticulocytes, is reliable as a short-term in vivo genotoxicity test: Summary of the MMS/JEMS-collaborative study across 16 laboratories using 24 chemicalsThe in vivo mutation assay using the X-linked phosphatidylinositol glycan class A gene (Pig-a in rodents, PIG-A in humans) is a promising tool for eva…
Examples to Pig-a limitations:
1) both tests were unable to identify many chemicals neurotoxicity, including AZT.
2) The RBCs results are time sensitive (better accuracy at week 4 vs week week 1)…
For how long did the Molnupiravir test run? we have NO IDEA!
Now that we put that aside, let us look at the response of Troth et al to Zhou et al paper, where they claimed that Zhou et al's results were incorrect due to multiple reasons.
Troth et al claims against Zhou et al paper:
1) Exposure was too long
2) Historical data was missing
3) cytotoxicity was not assessed at low dosage for 32 days
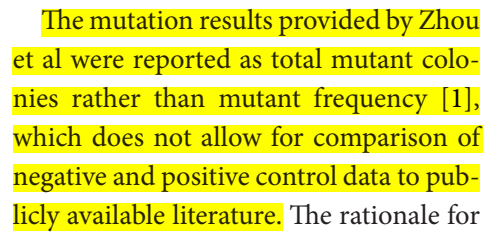
4) Zhou et al measured mutant colonies rather than mutant frequency.
51/x
SIDE NOTE: funny to see such claims in a letter that contains within it reference to two researches that are supposed to disprove safety concerns... but were privately conducted and did not disclose any information on practically anything.
#DualStandards?
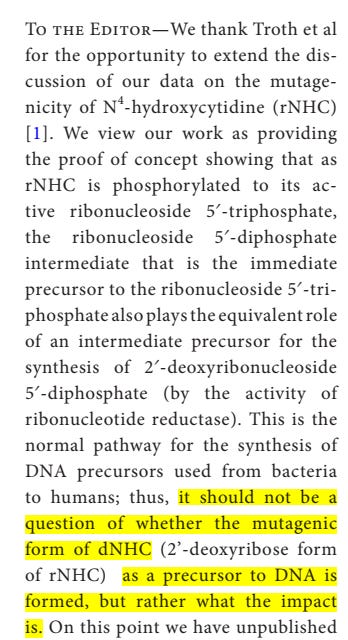
Zhou et al replied to Troth et al, claiming it should not be a question of whether molnupiravir is mutagenic or not, but how much.
https://doi.org/10.1093/infdis/jiab363
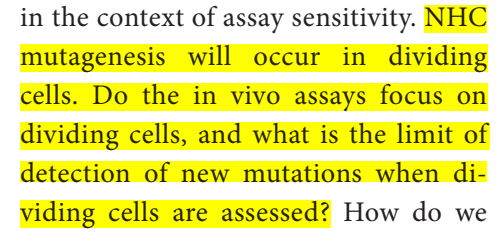
I will breakdown the reply of (and counter concerns raised by) Zhou et al:
Do the in-vivo assays focus on dividing cells, and what is the limit of detection of new mutations when dividing cells are assessed?
Because mutagenesis is revealed over a long period in cancer rates and germline mutations, how can Troth et al scale these negative results to a human lifespan?
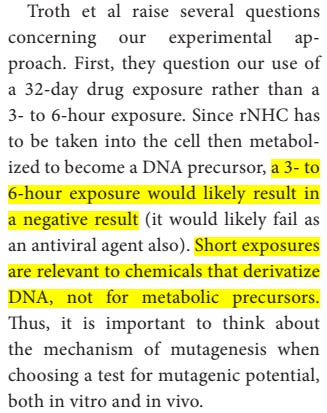
They explain the 32 days exposure was because a 3-to-6-hour exposure is not enough time for molnupiravir to be taken into the cell then metabolized to become a DNA precursor, and such a short timeframe experiment is not suited for metabolic precursors.
They defended not testing for cytotoxicity at low dosage for 32 days, and stated they "did not notice a difference in growth rate in the presence of 3 µM rNHC during the multiple rounds of cell passage".
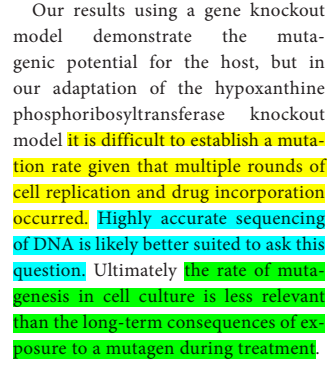
Because of the "multiple rounds of cell replication and drug incorporation", "it is difficult to establish a mutation rate", which is "is less relevant than the long term consequences of exposure to a mutagen during treatment."
They closed with:
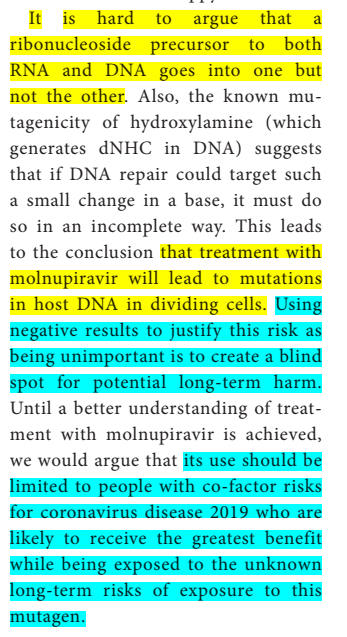
"It is hard to argue that (molnupiravir) a ribonucleoside precursor to both RNA and DNA goes into one but not the other… TREATMENT WITH MOLNUPIRAVIR WILL LEAD TO MUTATIONS IN HOST DNA IN DIVIDING CELLS".
and that "using negative results to justify this risk as being unimportant is to create a blind spot for potential long-term harm", and that because of that molnupiravir must only be given to people with cofactor risks bigger than mutagenic risks.
With this, I finalized the disproved² sub-thread.
Few more questions, before we end.
Why would regulators rely on assurances provided by private labs which were paid by the manufacturer, using study information that is unavailable to the academic community (and public) to review and scrutinize?
Why no one is raising questions whether or not the methods being used to provide these assurances (e.g. using pig-a assay) are fit-for-purpose and can truly measure with high accuracy the mutagenetic risks related to molnupiravir?
WHERE ARE THE JOURNALISTS? WHERE ARE THE RESEARCHERS? WHERE ARE THE REGULATORS?
The safety information provided for molnupiravir raises more questions than answers.
WE REACHED THE END of this thread which looked at molnupiravir safety.
AGAIN we see an attempt to push aside long-term concerns, AGAIN we see science being ignored, AGAIN we see how profit comes before our safety.
THIS IS IMMORAL. THIS IS WRONG. #RESIST!
#MERCKyBusiness
FINALLY...
IF YOU CAN, PLEASE CONSIDER SUPPORTING MY WORK, THANK YOU!
PayPal: paypal.me/ehdenbiber
Revolut: revolut.me/ehden
Bitcoin: bit.co.in/ehden
patreon.com/ehden
XRP: rUpmsXibCjtFpPaP4GmzRCuU4tx5UaP1Js



























Dear Ehden Biber
I would love to get in touch with you and invite you to present to the World Council for Health your findings. Please send me an email when you get a chance. I have just subscribed, so in the interest of privacy perhaps you can find it there. ...@worldcouncilforhealth.org
Many Thanks
Zoe
www.worldcouncilforhealth.org
To answer this question: "Why would regulators rely on assurances provided by private labs which were paid by the manufacturer, using study information that is unavailable to the academic community (and public) to review and scrutinize?" ... it is even worse than this.
Only pharma controls the details of any study. They paid for it; they own it. Even the regulators do not get access to it. The regulators get pharma's _interpretations_ of the data (and pharma can and does throw out data that doesn't look good). Unless and until there is a lawsuit (e.g., Vioxx after killing 40-60,000), all the data does not see the light of day. And even then, pharma will settle with the regulators in order to keep details hidden. Forever. See this podcast and we will want to read the book when it comes out in February. (Now. Consider that in light of the free passes that pharma currently has with the experimental jabs. WHEN will people wake up from this psychosis!?)
John Abramson, MD, is a Harvard Medical School Lecturer, national drug litigation expert, and author. His new book, "Sickening: How Big Pharma Broke American Health Care and How We Can Repair It," will be available on February 8, 2022.
https://open.spotify.com/episode/64ZsPU8e2CHvWQM9lqnLEY
Joe Rogan #1756 (free to listen/watch on Spotify)